rate of diffusion in ions lab report
advertisement

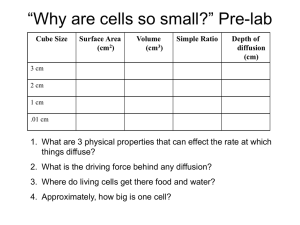
Chloe Troulan Investigating the Diffusion of Ions Introduction In order for cells to survive, they must constantly exchanged ions, gases, nutrients and wastes with their environment. These exchanges take place at the cells surface. In this lab activity you will use bouillon cubes, which have a high salt content, as models of ion diffusion in water. You will observe how the rate of diffusion is affected by the addition of increasing the amounts of bouillon cubes. When the bouillon cubes are placed in the distilled water, they will begin to dissolve, releasing sodium and chloride ions. The solutions conductivity, measured by a conductivity probe, is proportional to the ion concentration in the solution after diffusing from the cube. Research Question: How is the rate if the ion exchange affected by the increase in the concentration of ions? Hypothesis: If concentration of ions is increased by adding bouillon cubes then the rate of ion exchange will increase proportionally. Materials: - Data Logger - Conductivity Probe - Distilled Water - 3 X 100ml Beakers - Bouillon cubes - utility clamp - Ring Stand - Computer Procedure: 1. Set up the utility clamp, conductivity probe and ring stand 2. Set the selector switch on the conductivity probe to the 0-20,000 us/cm range 3. Change the data collection rate to 0.5 samples/second and data collection length to 360 seconds 4. Pour 80ml of distilled water into the 400ml beaker. Position the probe in the water 3cm from the bottom of the beaker close to one end of the beaker. 5. Place one solid bouillon cube in the beaker furthest away from the probe and star the data collection. Do not stir the solution. 6. After the time has run its course analyze the graph to determine the rate of ion exchange. a) Examine the graph and identify the most linear region. b) Highlight those data points. c) Select linear fit under analyze menu. d) Record the slope, m, as the rate of ion exchange. 7. Rinse the conductivity probe thoroughly. 8. Repeat with an additional 2 beakers of the same setup. 9. Rinse the conductivity probe and beakers thoroughly. Dry the beakers and probe using a paper towel. 10. Repeat steps 4-9 for two bouillon cubes placed in the same area. 11. Repeat steps 4-9 for 3 bouillon cubes placed in the same area. Data/ Results: Table 1 Rate of Ion Exchange 1 Bouillon Cube (us/cm) Trial 1 161.1 Trial 2 129.1 Trail 3 132.7 2 Bouillon Cubes (us/cm) 198.9 185.4 215.5 3 Bouillon Cubes (us/cm) 256.2 228.0 233.1 Diffusion in Ions 300 Rate of Ion Exchange 250 200 Trial 1 150 Trial 2 100 Trial 3 50 0 1 Bouillon Cube 2 Bouillon Cubes Number of Bouillon Cubes Used 3 Bouillon Cubes The means of the data above are: 1 Bouillon cube – 140.966667 um/cm 2 Bouillon cubes – 199.933333 um/cm 3 Bouillon cubes – 239.1 um/cm Conclusion From looking at the results in the table above we can come to the conclusion that once the surface area of the Bouillon cubes was increased we saw that the rate of ion exchange increased as well. Although the ion exchange increased the rate of diffusion on the ions decreased, this was because when one bouillon cube was placed in the water it took about 140.966667 um/cm compared to when 2 bouillon cubes were added and it took about 199.933333 um/cm. This is because it takes longer for the salt to diffuse from the inside to the outside when there is a larger surface area, this is because there is more material to travel through. So the main conclusion is that because cells are continuously exchanging their ions, nutrients and wastes and also nutrients from their surface this means that this process slows down due to the increase in surface area. When we look at the actual size of cells we see that there is a purpose why they are so small because when they are too large the surface area affects the rate of processes in the cell. Limitations There were very few limitations in this lab because it was pretty well thought out and also before we went through and did the experiment we went through and changed different variables that were stated in the original pre-lab. I would say that there may have been one main limitation that I could pick out and that would be the temperature of the water, this I would think may affect the rate of diffusion in some way, the only way I could find this out would be to re-do the experiment with the same temperature of water in each beaker. Modifications There would probably be a few modifications that I would add and that would be to add more variables such keeping the water temperature the same and adding more variables.