Wordfile
advertisement

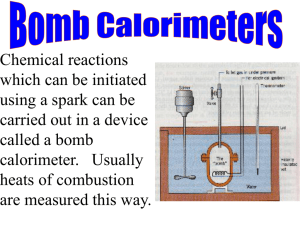
CHE 3200 HEAT OF COMBUSTION 2015 OBJECTIVE: (a) To determine the molar heat of combustion of naphthalene (b) To perform an error analysis on your determination. DH N (c) To find the error in the determination which contributed most to the error in (e) To compare your value of DH N DH N with the literature value. BACKGROUND: Determination of the Heat of Combustion of solid Naphthalene : We are concerned with determining experimentally the change in enthalpy per mole of the combustion of solid naphthalene. The reaction is : C10H8(s) with : + 12 O2(g) Dngas = -12 +10 = -2mol DH = We will call this DH n n= DH = m M and 10 CO2(g) Dngas n = -2 + 4 H2O(l) where n = 1 mol C10H8(s) molar change in enthalpy DH comb Notation used : = DH N DH m therefore : molar heat of combustion (units : kJmol-1) specific change in enthalpy (units : kJ g-1 ) DH = DH × M = DH × M m in words : the molar heat of combustion is equal to the specific heat of combustion times the molar mass. n = number of moles m = mass M = molar mass H = enthalpy U = internal energy Solid naphthalene will be pressed into a pellet and a 10 cm piece of iron wire will be fused into the pellet. The combustion of the iron wire will have to be taken into account. The reaction will be carried out in a “Bomb Calorimeter” which consists of a rigid container with diathermal walls (the bomb) seated in a pail of water enclosed in adiabatic (or nearly adiabatic) walls. The system is the inside of the bomb and does not include the walls of the bomb. The surroundings are basically the walls of the bomb and the pail with water (the whole calorimeter minus the contents of the bomb). The surroundings plus the system is everything inside the 1 adiabatic walls, so the surroundings plus the system are the equivalent of the universe as it is usually discussed. The combustion reaction will take place inside the bomb (in the system) and will be exothermic. Heat will be produced and will leave the system and enter the surroundings, so : qsurroundings = -qsystem The temperature change of the surroundings will be recorded qsurroundings = C × DT with D T = (T2 – T1 ) C = heat capacity of the surroundings qsystem = DU N mN + DUFemFe N = naphthalene , Fe = iron wire C(T2 - T1 ) = -(DU N mN + DUFe mFe ) DU N remember : DH = DU N But we want = [- C(T2 - T1 ) -DUFe mFe ] DH × M = DH × M m = DU N M N DH N = = [- DH N n 1 mN C(T2 - T1 ) -DUFe mFe ] ( not remember from General Chemistry : (1) DU N (2) MN mN (3) ) H = U + PV DH = DU + D(PV) = DU + D(nRT) = DU + RTDngas DH = DU + D(PV ) = DU + RT Dngas n DH N = [- Dngas n = -2 C(T2 - T1 ) -DUFe mFe ] MN -2RT mN DUFe, M N , R Quantities measured : mFe, mN ,T1,T2 ,T = T1 Quantities known : Quantity to be determined : C C will be determined in Part A of the experiment (week 1) 2 (4) Determination of the heat capacity C of the “surroundings” : C will be determined using the combustion of a measured mass of a substance (benzoic acid , BA) of which we know the specific internal energy of combustion using equation (1) above : for BA : it follows : DU BA C(T2 - T1 ) = -(DU N mN + DUFe mFe ) C(T2 - T1 ) = -(DUBA mBA + DUFemFe ) (5) -(DU BA mBA + DUFe mFe ) (T2 - T1 ) (6) C= METHOD: PART A (week 1) : A Parr Bomb Calorimeter with a double valve bomb will be used to determine the heat capacity C of the “surroundings” . Prepressed pellets of benzoic acid will be available. A 10 cm piece of iron wire will be fused into the pellet. The mass of the BA pellet and the Fe wire will be determined. The change in temperature of the “surroundings” will be determined using a LabQuest2 and the LoggerPro program. Two determinations will be performed in week one of the experiment and the average C will be used for part B. PART B (week 2) : The same calorimeter will be used to determine the molar heat of combustion of naphthalene. A pellet of naphthalene will be pressed using a pellet press. Iron wire will be fused into the pellet. The mass of the naphthalene and the Fe wire will be determined. The change in temperature of the “surroundings” will be determined using a LabQuest2 and the LoggerPro program. Two determinations will be performed in week two of the experiment and the average reported. PROCEDURE: The procedure will be discussed in Recitation (10/14/2014 and 10/16/2014). 3 DH N will be CALCULATIONS: Part A : Calculation of C : DU BA is given on the flask containing the pellets. (Note the units and the sign. The sign is not given on the flask). DUFe is given on the cardboard on which the iron wire is wrapped. (Note the units and the sign. The sign is not given in the cardboard). mBA and mFe are determined using the analytical balance in the lab. (Use the balance with the most decimal places). T1 and T2 and the errors e(T1) and e(T2) are determined using LoggerPro. Example T2 and e(T2) : Highlight the linear portion of the graph after the maximum of the curve. Click linear fit (on the top bar). A little menu will appear which contains m, b, Correlation, and RMSE. Use m and b to find T2 (x = ignition time, y = T2). Use the RMSE for the error in T2. Use equation (6) , two determinations. Take the average C av Note : DU BA and DUFe are both < 0 , so C>0 Part B : Calculation of DH N : DUFe mN is given on the cardboard on which the iron wire is wrapped and mFe are determined using the analytical balance in the lab. T1 and T2 and the errors e(T1) and e(T2) are determined using LoggerPro (see above). Use equation (4) , use the average Cav , T = T1 Two determinations. Report the average. ERROR ANALYSIS : Use equation (6) for the propagation of the error to C. e(C) has six terms. Use equation (4) for the propagation of the error in DH comb,N . e( DH comb,N ) has eight terms. DISCUSSION : Don’t forget to discuss which of the errors contributed most to the error in the molar heat of combustion of naphthalene. You also need to show this. Don’t forget to compare your value to the literature value. 4