Identifying Recessive Laminin Gene Mutations in Zebrafish
advertisement

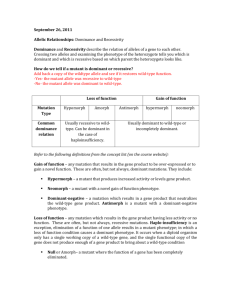
Identifying Recessive Laminin Gene Mutations in Zebrafish Kaylin Pound Kp27402p@pace.edu Biology 231: Genetics Dr. Horne Pace University: Department of Biology Abstract: The Laminin gene is an important gene in zebrafish that plays a vital role in embryonic development. In this experiment forward genetics was used to examine mutations in this gene and understand how defects compromise functions carried out by Laminin proteins. The mutation under consideration was caused by a random viral insertion into genome of some of the zebrafish embryos, resulting in the alteration of the Laminin C gene. The mutation resulted in the production of nonfunctional Laminin proteins, which ultimately caused a curved tail phenotype in the embryos as opposed to the normal straight tail phenotype. The objective of this experiment is to determine if the mutation is caused by a dominant or recessive allele. An additional goal of this experiment is to identify which of the normal and mutant embryos contain the viral insert that is responsible for the mutation. In order to achieve these objectives, we mated two adult zebrafish, referred to as Lary, and studied their progeny. A quantitative assessment was carried out on the offspring to determine the ratio of embryos displaying the normal phenotype to the mutant phenotype. In turn, this would allow us to determine which phenotype is dominant and which phenotype is recessive. Following this, PCR tests were conducted on the normal and mutant embryos as well as a positive and negative control samples to identify which embryos contained the viral insert. The first part of the experiment revealed that 73% of the progeny of the Lary x Lary cross exhibited the normal phenotype while 27% exhibited the mutant phenotype. From this information, it is clear that the mutation was caused by a recessive allele. This conclusion was further supported by carrying out a cross between a homozygous dominant individual (wild type) with a heterozygous individual. The offspring of this cross displayed a 100% normal phenotype, thus confirming the idea that the normal phenotype is dominant and the mutant phenotype is recessive. Further testing through the use of PCR revealed that the viral insert was present in both mutant samples that were tested and one of the 4 normal samples that were tested. Introduction: The zebrafish, Danio rerio, serves as a good model organism for investigating genetics and development due to the fact that they are vertebrates with short generation times and have transparent external embryos that can be easily manipulated (Twyman). Upon mating 2 adult zebrafish from the Lary line, offspring were produced that displayed both normal embryos as well as mutant embryos, which were easily recognizable by differences in their morphology (Horne 1). This mutant phenotype produced curved tails within the progeny and resulted from a genetic mutation possessed by the Lary adults. By quantitatively analyzing the progeny of this cross to identify the ratio of normal to mutant embryos, a determination of whether the mutant phenotype is dominant or recessive can be made. Furthermore, this data can also be used to predict the genotypes of the parents that were crossed to produce the progeny under consideration. The curved tail phenotype displayed by the progeny was produced by a loss of function mutation in the gene for Laminin (Horne 2). This gene produces cytoskeleton proteins that are essential to notochord development and the structural integrity of tissues and of the nucleus within the organism (Stemple). The notochord plays an essential role in the development of vertebrates and serves as an axial skeleton for embryos, which is critical for locomotion. The loss of functional Laminin proteins affects notochord development by altering the formation of the peri notochordal basement membrane, which ultimately produces embryos with a severe downward curvature (Stemple). This experiment utilized forward genetics to examine mutations from altering the Laminin C gene in zebrafish. Forward genetics, involves the random mutation of a gene, screening mutations to find one that mutates a certain function and going back to identify which gene was mutated. A viral vector was used to produce the Lary mutation in which an insertion of a portion of the viral genome into the middle of the coding sequence for the Laminin C gene occurred. The extra codons present in the Laminin C gene ultimately leads to the production of the nonfunctional Laminin proteins, which serves as the molecular defect responsible for the mutant curved tail phenotype. A polymerase chain reaction (PCR) test can be used to detect the presence of the mutant Laminin gene, therefore revealing which of the mutant and normal embryos contain the viral insertion. Polymerase Chain Reaction (PCR) is an enzymatic in vitro method for rapidly amplifying specific segments of DNA (Peirce). PCR requires primers that are made to either end of the sequence of target DNA to be amplified where the forward primers initiate the replication of one strand, while the reverse primers initiates the replication of the complementary strand in the opposite direction (Horne 2). The cycling of specific temperatures which include heating to separate DNA strands, cooling to allow short primers anneal to the target DNA, and reheating to extend the new DNA strand with a polymerase leads to the exponential production of the target DNA sequence between the two primers (Peirce). This process can used to determine the presence of the mutant allele in the zebrafish by using a forward primer that recognizes a sequence in the viral insert and a reverse PCR primer that recognizes a sequence in the zebrafish Laminin gene. If the mutant allele is present, the PCR reaction will result in the production of many copies of the sequence between the viral insert primer and the Laminin gene primer, producing a band that is 245 bp long (Horne 2). However, if the mutant allele containing the viral insert is not present, no PCR product will be made because the forward primer will not be able to bind. The presence of the 245 bp product can be determined by running the reaction mixture on agarose gel and comparing the product to DNA molecular weight markers. The arrangement of the amino acids in the Laminin gene plays a significant role the structure of the protein and therefore how the protein functions to express the gene. When comparing the expression of the Laminin gene between zebrafish and humans, the sequence of amino acids is approximately 70% similar whereas 30% of the gene is arranged differently or has gaps that do not contain the same codons. Since zebrafish and humans are vertebrates that display close homology, studying mutations of the Laminin gene in these organisms can be useful to the understanding of human diseases such as muscular dystrophy (improper muscle formation), junctional epidermolysis bullosa (skin blistering disease) and nephrotic syndrome (defective kidney filter) caused by mutations in this gene (McGowan). Materials and Methods: The first step in the experiment was to mate 2 adult Lary zebrafish, in which a male is placed on one side of the mating chamber and a female is placed on the other side. The fish are usually left separated by a divider in the mating chamber overnight so they can recover from the stress of being moved and get acquainted to their new surroundings. The tank was placed in a room that had a light cycle of 14 hours on and 10 hours off. The fish mate at lights on so the divider was removed at 10 am on Saturday so the exact age of the embryos could be determined. The embryos were then collected with a strainer and placed in a 28°C incubator for 72 hours. Next we placed the embryos in a petri dish containing the embryo media and examined them using a dissecting microscope. A separate petri dish containing a drug that stopped the embryos from swimming was also made available to us. We identified the mutant curved tail phenotype among the progeny and counted the number of normal and mutant offspring in the progeny by splitting the embryos up into several dishes and repeated this counting process under the microscope and with the naked eye to confirm that our numbers were accurate. We then used this data to hypothesize the genotypes of the parent Lary’s and constructed a Punnett square of this cross to support our hypothesis. A chi square analysis was also done to further support our speculations regarding the genotype of the parent Larys. In addition, we also came up with another possible cross that could be used to support our hypothesis of the parent’s genotypes. This cross was confirmed by quantitatively analyzing the progeny that resulted from mating a homozygous dominant, wild type individual with the heterozygous Lary in the same manner that the two Lary individuals were mated. The next part of the experiment involved the use of PCR (Polymerase Chain Reaction) to determine the presence or absence of the mutant allele within the embryos. The first step of this was DNA Extraction, in which we placed 3 mutant embryos and 5 normal embryos into petri dishes containing egg water and tricaine. The fish were then euthanized by placing the dishes on ice for 15 minutes. Next we added each embryo to a separate 1.5 mL microcentrifuge tube and labeled them with either “mutant” or “normal.” Any excess buffer in the tubes was removed with a 200-microliter pipet to ensure accurate PCR results. Next, 50 ul of Extraction Buffer was added to each tube, and the tubes were incubated at 55° C for 15 minutes, vortexted and the incubated 55° C for another 15 minutes. After this, the tubes were incubated at 95° C for 10 minutes. Next, 150 ul of water was added to the tubes and the tubes were vortexed again. The contents of the tubes contained the DNA to be used for the PCR reaction. To carry out the PCR reaction we first placed the DNA samples into 0.5 mL tubes. In each of the tubes we put 19 ul water, 2 ul Laminin Primer, 2 ul Viral Primer, 2 ul of the DNA sample, and 25 ul of PCR Master Mix. We labeled all of the tubes with “normal” or “mutant” accordingly and mixed them. In addition, we included a “negative control” tube that consisted of water to ensure that no contamination occurred during the process, and a “positive control” tube that contained DNA with the viral mutation to ensure that the DNA polymerase was working properly. Then we placed them in the PCR machine and let them go through the cycle. In the PCR program, the samples were at 94° C for 2 minutes. Then, it was at 94° C for 15 seconds, 60° C for 30 seconds, and 72° C for 1 minute. It went through this step 33 times before the final step, which was at 72° C for 5 minutes. After this cycle was complete, the samples were frozen for one week. The next part of the lab involved analyzing the PCR product by Agarose Gel Electrophoresis. This was done by creating a 1% agarose solution in 100 mL of TAE buffer. The solution was melted in the microwave for approximately 30 seconds, and then cooled for 10 minutes. Next, 3 ul of DNA dye was added to the gel which enabled us to determine the how far the products traveled down the gel when the gel electrophoresis stopped running. Then, we poured the gel into the box and allowed it to settle for a few minutes until it was ready to begin to electrophoresis. In order to run the gel, we first placed 15 ul of each of the PCR reaction mixtures and 3 ul of gel sample buffer into separate small microcentrifuge tubes. In a separate tube, we added 10 ul of DNA standards and 3 ul of the gel sample buffer. We placed 15 ul of each of these mixtures into the wells of the agarose gel and ran the gel at 100 volts for 30 minutes. After this, we examined the results under a UV to determine which samples showed PCR products of the appropriate 245 bp length. We were able to confirm these particular samples contained the viral mutation of the Laminin gene due to the fact that the samples in which the viral insert was absent did not produce any PCR product since the viral primers had nothing to bind to in the target DNA. Results: For the first part of the experiment, we quantitatively assessed the progeny of the Lary x Lary cross by counting the number of straight tailed (normal) zebrafish versus the number of curved tailed (mutant) zebrafish (See Fig.1). There were 116 embryos in total and we found that 85 embryos displayed the normal phenotype while 31 embryos displayed the mutant phenotype. Next we constructed a Punnett square that reflected phenotypic ratios similar to the numbers we obtained by counting the progeny (Fig. 2). From this we formulated a hypothesis that the cross must have been between two heterozygous parents and the mutation must be recessive. To further test our hypothesis, we set up a cross between a heterozygote and a homozygous wild type individual. Upon constructing a Punnett square for this cross we were expecting to see a progeny that displayed 100% normal straight tailed embryos with the dominant phenotype and 0% mutant recessive phenotype (Fig. 3). After examining the offspring of this cross we did in fact find that the progeny all displayed the normal phenotype for straight tails. To determine the accuracy of our results we performed a chi square analysis in which we compared the percentage of observed phenotypes from the samples we examined to the expected phenotype percentages given by the Punnett square. The chi square value of our results was 0.19 with one degree of freedom. Since our chi square value fell between the 80-85% range of what was expected, our observations did not occur due to some chance event. Therefore, the null hypothesis is accepted and the data supports our hypothesis. Phenotype Observed Expected (o-e) (o-e) 2 (o-e) 2 /e Normal Straight Tails (N) Mutant Curved Tails (n) 85 87 2 4 0.05 31 29 -2 4 0.14 Total = 0.19 Fig 4: Chi Square Analysis For Lary x Lary cross In the PCR section of the experiment, we ran a test on the DNA of the mutant embryos and the normal embryos. A test sample was placed in the first well, followed by the positive control sample, the negative control sample, an empty well and then the normal embryo samples in the remaining 4 wells. The positive and negative control samples allowed us to determine that no contamination occurred during the process and that the DNA polymerase was working properly. We ran the gel and found one normal sample that contained PCR product of the appropriate 245 bp length (Fig. 5). We ran an additional PCR test in which 2 of the samples for the mutant embryos were used in place of the normal embryos. Upon looking at the results, we found that PCR product of the correct 245 bp length was produced for both of the mutant embryo samples (Fig. 6). Fig 5 represents the products in which the base pairs from the Polymerase Chain Reaction were amplified. Each letter indicates what sample was put in each well. A test sample was placed in the first well (S) that showed where all of the base pairs were located, followed by the positive control sample (+), the negative control sample (-), an empty well (X) and then the normal embryo samples (N) in the remaining 4 wells. One of the normal embryos displayed the 245 bp PCR product. Fig 6 represents the products in which the base pairs from the Polymerase Chain Reaction were amplified. Each letter indicates what sample was put in each well. A test sample was placed in the first well (S) that showed where all of the base pairs were located, followed by the positive control sample (+), the negative control sample (-), 3 empty wells (X) and then the mutant embryo samples (M) in the remaining 2 wells. The positive control and both mutant embryos displayed the 245 bp PCR product. Discussion: Upon looking at the progeny of the first cross (Lary x Lary) we were able to formulate a hypothesis regarding the genotypes of the adult Lary fish. We predicted that these adults had a heterozygous genotype in which the normal phenotype allele was dominant over the recessive mutant allele. The results of the chi square analysis showed that our data was able to support our hypothesis. A Punnett square showing the cross between two heterozygous Lary adults displayed a 3:1 ratio of the normal dominant phenotype to the mutant recessive phenotype. This indicates that 75% of the progeny should have displayed the normal straight tail phenotype, while the remaining 25% of the progeny should have displayed the mutant curved tail phenotype. Upon examining our progeny we found that 73% of the offspring showed the normal phenotype while the remaining 27% of the offspring displayed the mutant phenotype. The deviations from the expected percentages can most likely be attributed to human error in counting the number of individuals displaying each phenotype or using a sample of progeny that was not large enough. Since the chi-square value showed that we were within 80-85% of our expected data, we were able to conclude that the straight tailed phenotype is dominant to the curved tailed mutation. After examining the progeny of the second cross (Wild Type x Lary), we found that all of the progeny displayed normal phenotypes which was conducive with the expected ratios given by Punnett square for this cross. This allowed us to reconfirm the idea that the Lary adults had a heterozygous genotype and that the Wild Type individuals had a homozygous dominant phenotype. In this cross, the heterozygous Lary adult was able to pass on one recessive mutant allele, however the homozygous dominant Wild Type lacked the recessive allele that would be necessary for the production of offspring with the mutant (nn) recessive phenotype. The fact that all of the offspring exhibited a 4:0 phenotypic ratio of normal to curved tails also reconfirms the idea that the allele for normal tails is dominant over the allele for curved tails. Had the mutation been dominant, all of the progeny of the second cross would have exhibited the mutant curved tail phenotype. This experiment utilized forward genetics to examine mutations that arise from altering the Laminin C gene in zebrafish. Forward genetics, involves the random mutation of a gene, screening mutations to find one that mutates a certain function and going back to identify which gene was mutated. The mutant allele responsible for the curved tail phenotype in the Lary progeny was produced by a viral insertion that altered the base pair sequence of the Laminin C gene in the zebrafish genome. As a result of this, we predicted that only the offspring that possessed the recessive allele for mutant phenotype should contain the 245 bp mutant Laminin gene that contains the viral insert. In the second experiment, we confirmed our hypothesis by conducting PCR tests utilizing the viral and normal primer of the Laminin gene to detect the presence or absence of the desired 245 bp PCR product. Upon running the gels we expected to obtain results in which all of the mutant embryo samples showed the 245 base pair PCR product. After analyzing our PCR gels we found that only the positive control and mutant embryo samples showed the desired PCR product. The positive control sample contained DNA with the confirmed viral insert. Therefore, the PCR product produced by the positive control showed that the DNA polymerase was working properly in the reaction. The PCR product produced by the mutant embryo samples was consistent with our hypothesis and showed that the mutant embryos contained the 245 bp viral insert in the mutated Laminin C gene that we were attempting to amplify. Upon examining the gels of the normal embryos, we found that one of our four normal samples produced PCR product. The Punnett square indicated that 2/3 of the normal progeny should have contained one recessive allele in which the viral insert was present and to which the primers could anneal to the complementary sequence. The deviation between our results and the expected results could have arisen as a result of a pipetting error or not using enough normal embryo samples to make an accurate determination. From this lab we were able to conclude that the Laminin gene is vital for the proper development of zebrafish embryos. The Laminin gene is responsible for producing cytoskeletal proteins, which are essential to notochord development as well as the structural integrity of the nucleus and various tissues. A viral insertion in the Laminin gene such as the one observed in the mutant Lary individuals results in a loss of function mutation thus producing nonfunctional Laminin proteins. The absence of functional Laminin proteins severely compromises the proper elongation of embryos as well as locomotion. The alteration in the morphology of the embryos resulting from this loss of function mutation is typically fatal thus highlighting the importance of the Laminin gene in the structure and survival of the embryos. References: 1. Horne, Jack. Mutant Zebrafish Experiment (Handout #1). 2. Horne, Jack. PCR Genotyping Lab (Handout #2). 3. Mcgowan, Kelly Ann, and M. Peter Marinkovich. "Laminins and Human Disease." Microscopy Research and Technique 51.3 (2000): 262-79. Print. 4. Pierce, Benjamin A. Genetics: A Conceptual Approach. Basingstoke: Palgrave Macmillan, 2011. Print. 5. Stemple, D. L. "Structure and Function of the Notochord: An Essential Organ for Chordate Development." Development 132.11 (2005): 2503-512. Print. 6. Twyman, Richard. "Model Organisms: Fish." The Human Genome. Wellcome Trust, 29 Aug. 2002. <genome.wellcome.ac.uk/doc_WTD020806.html>.