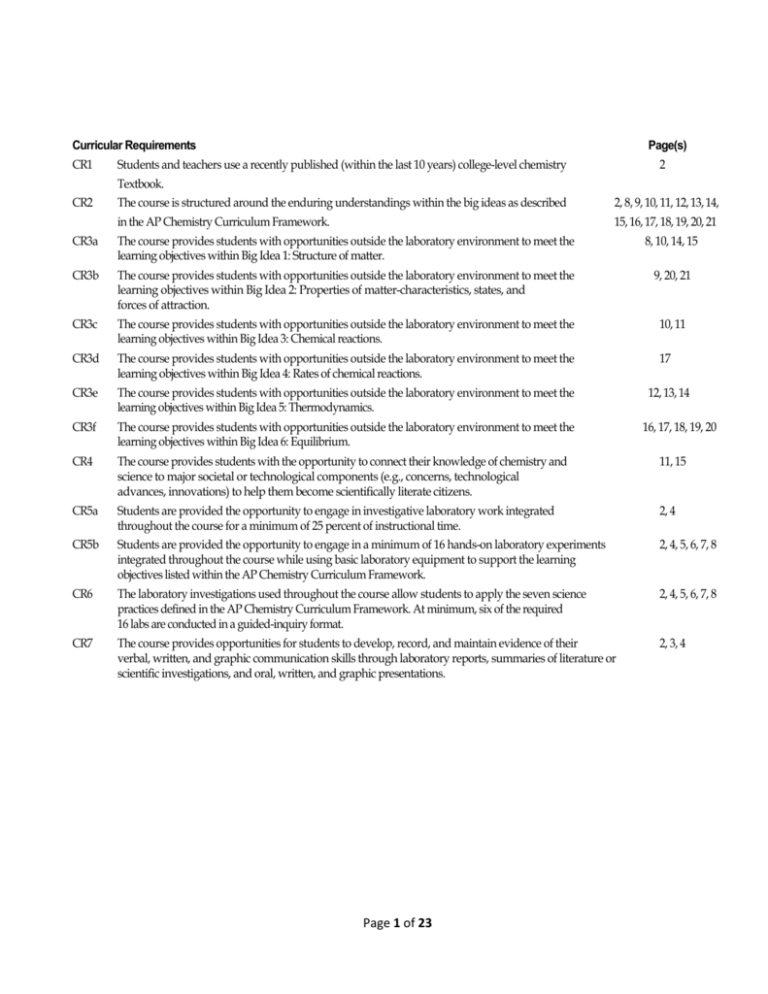
Curricular Requirements
CR1
Page(s)
Students and teachers use a recently published (within the last 10 years) college-level chemistry
2
Textbook.
CR2
The course is structured around the enduring understandings within the big ideas as described
2, 8, 9, 10, 11, 12, 13, 14,
in the AP Chemistry Curriculum Framework.
15, 16, 17, 18, 19, 20, 21
CR3a
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 1: Structure of matter.
8, 10, 14, 15
CR3b
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 2: Properties of matter-characteristics, states, and
forces of attraction.
9, 20, 21
CR3c
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 3: Chemical reactions.
10, 11
CR3d
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 4: Rates of chemical reactions.
17
CR3e
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 5: Thermodynamics.
CR3f
The course provides students with opportunities outside the laboratory environment to meet the
learning objectives within Big Idea 6: Equilibrium.
CR4
The course provides students with the opportunity to connect their knowledge of chemistry and
science to major societal or technological components (e.g., concerns, technological
advances, innovations) to help them become scientifically literate citizens.
11, 15
CR5a
Students are provided the opportunity to engage in investigative laboratory work integrated
throughout the course for a minimum of 25 percent of instructional time.
2, 4
CR5b
Students are provided the opportunity to engage in a minimum of 16 hands-on laboratory experiments
integrated throughout the course while using basic laboratory equipment to support the learning
objectives listed within the AP Chemistry Curriculum Framework.
2, 4, 5, 6, 7, 8
CR6
The laboratory investigations used throughout the course allow students to apply the seven science
practices defined in the AP Chemistry Curriculum Framework. At minimum, six of the required
16 labs are conducted in a guided-inquiry format.
2, 4, 5, 6, 7, 8
CR7
The course provides opportunities for students to develop, record, and maintain evidence of their
verbal, written, and graphic communication skills through laboratory reports, summaries of literature or
scientific investigations, and oral, written, and graphic presentations.
2, 3, 4
Page 1 of 23
12, 13, 14
16, 17, 18, 19, 20
CHEMISTRY AP
GENERAL CURRICULAR REQUIREMENTS
INSTRUCTIONAL OBJECTIVES: The AP Chemistry course is designed to be the equivalent
of the general inorganic chemistry course usually taken during the first year of college. For some
students, this course enables them to undertake, as freshmen, second-year work in the chemistry
sequence or to register in other courses where the general chemistry course is a prerequisite. The
AP Chemistry course should be rigorous enough to contribute to the development of the
students’ abilities to think clearly and to express their ideas logically and with clarity. The course
covers chemistry topics more in depth and with greater emphasis on chemical calculations,
mathematical formulation, and the theoretical aspects involved in chemistry. (College Board,
2012).
The AP Chemistry course is designed and conducted to give the student a deeper understanding
in chemistry. Students are expected to take the AP Chemistry exam given in May of each year.
Passing this exam may allow the student to enter the college chemistry sequence at a knowledge
level above that of the normal student. Students are expected to work at levels higher (college
level) than those found in a regular class, including conducting advanced chemistry laboratories.
Additionally, intense studying outside of the regular class will be required for the student to be
successful. Even though students will be working on college level material, it is advantageous to
complete certain courses in high school because teachers are able to tutor and encourage in a
manner not usually found in college.
COURSE DESIGN: Students taking AP courses are required to make interdisciplinary
connections. Due to the large number of connections between chemistry and physics, students at
our school are required to take both the AP Chemistry course and the AP Physics course
concurrently. The students are also required to take a separate AP laboratory class that meets
every other day where AP Chemistry and AP Physics laboratories are alternated for the full year.
The laboratories are correlated to match the lecture concepts throughout the year. This three
course package helps the students make the connections between the curricula and the real
world. Additionally, the required lab class allows the students to complete over thirty chemistry
and thirty physics laboratories each during the school year. Laboratories are conducted by
students as individuals or in student centered groups requiring critical thinking of two to four
students.
TEXTBOOK: Chemistry, Zumdahl and Zumdahl, (publisher Brooks/ColeCengage) 9 th Edition
– AP Edition © 2013 [CR 1]
LECTURE COURSE: The lecture course meets for 90 minutes every other day for a total of
240 minutes in a 10 day block. The lab course also meets for 240 minutes in each block with a
minimum of 120 minutes being for chemistry resulting in approximately 33% of the contact time
being in the lab setting. [CR5a,b, CR6, CR7]
Lecture is taught using a combination of interactive lectures, discussions, and problem solving.
As part of the lecture, quizzes are periodically used to assess student comprehension and to
review and reinforce the taught concepts. Note taking and problem solving skills are constantly
stressed to help prepare the student for college. Comprehensive exams with both multiple choice
and free response questions are given at the end of each major topic. Released College Board AP
Chemistry questions and materials are used as part of every lecture. Students are required to
solve complex mathematical problems (i.e. Hess Law, Nernst Equation, Van der Waals
Equation, Ideal Gas Law, Rate Law, etc.). Students must also link problems and topics together
both mathematically and conceptionally. Problems from released AP exams are used as class
problems, review problems, and similar problems are placed on exams. The students complete
homework assignments for each lecture topic using the University of Texas Quest electronic
Page 2 of 23
question system. The Quest assignments each require from one to three hours for completion.
Teacher-created handouts and outlines are used to assist the students. The AP Chemistry Lecture
course is graded with major exams comprising approximately 70% of the grade and other
assignments (i.e. homework, class work, quizzes, etc.) counting approximately 30%.One hour
tutoring sessions are available before and after school three to five times per week. Additional 2
to 3 hour long tutoring sessions are scheduled during the spring semester as needed. Three day
long Saturday tutoring sessions are used each year to help review for the AP exam.
STRUCTURE OF THE COURSE: AP Chemistry is built around six big ideas and seven
science practices. The big ideas are: [CR 2]
Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter
can be understood in terms of arrangements of atoms. These atoms retain their identity in
chemical reactions.
Big Idea 2: Chemical and physical properties of materials can be explained by the structure and
the arrangement of atoms, ions, or molecules and the forces between them.
Big Idea 3: Changes in matter involve the rearrangement and/or reorganization of atoms and/or
the transfer of electrons.
Big Idea 4: Rates of chemical reactions are determined by details of the molecular collisions.
Big Idea 5: The laws of thermodynamics describe the essential role of energy and explain and
predict the direction of changes in matter.
Big Idea 6: Any bond or intermolecular attraction that can be formed can be broken. These two
processes are in a dynamic competition, sensitive to initial conditions and external perturbations.
The science practices for AP Chemistry are:
Science Practice 1: The student can use representations and models to communicate scientific
phenomena and solve scientific problems.
Science Practice 2: The student can use mathematics appropriately.
Science Practice 3: The student can engage in scientific questioning to extend thinking or to
guide investigations within the context of the AP course.
Science Practice 4: The student can plan and implement data collection strategies in relation to a
particular scientific question
Science Practice 5: The student can perform data analysis and evaluation of evidence.
Science Practice 6: The student can work with scientific explanations and theories.
Science Practice 7: The student is able to connect and relate knowledge across various scales,
concepts, and representations in and across domains.
LABORATORY INVESTIGATIONS: The laboratory portion of this class is to be the
equivalent of a college laboratory experience. As with most colleges, we have separate lecture
and laboratory classes. Because some colleges require proof of the laboratory portion of the
course before granting credit, all students will keep a laboratory binder. The laboratory binder
given to the students is designed to be a complete laboratory experience for the student. When
the students finish AP Chemistry, they are encouraged to take their laboratory binder with them
to college. [CR7]
The laboratory binder includes all thirty-one of the laboratory investigations to be completed
during the AP laboratory course. They are twenty-eight are “wet labs” and seven of the
laboratories are student inquiry based. Laboratories are selected from college laboratory
manuals, commercial laboratory manuals and commercial laboratory pre-designed kits. Students
are required to use critical thinking and analysis skills during all laboratories. When possible,
complex labs covering multiple topics will be used.
The school is on a block schedule, so class length is approximately 90 minutes, which generally
allows enough time for students to complete the laboratory investigation. However, the AP
laboratory course is scheduled for the last period each day, so the students know that if there are
Page 3 of 23
equipment problems, requiring extra time, they can stay during tutoring time to complete them.
Chemistry lab time comprises approximately 33% of contact time. Students must turn in
completed laboratory write-ups after each lab. Each student will be required to formally report
their results once per semester using a method of their choice (PowerPoint, Poster, Article, etc.) .
Additionally, at least once each grading period, class data will be compiled prior to completing
the lab report. [CR 5a, 5b, 6, 7]
LABORATORY EQUIPMENT: The school is equipped with a full range of glassware
(beakers, flasks, burets, eudiometer, pipets, etc.), instruments (Spec-20s, analytical balances,
centrifuges, ovens, etc.), and data gathering probes. All of the students have access to computers
with a full range of MS Office products on them. In addition, all computers have the Vernier
Logger Pro for data analysis. Data will be collected (1) by the students, (2) via computer or (3)
via data gathering handheld units. All data is recorded in their laboratory binder. [CR 5b]
LABORATORY INVESTIGATION SEQUENCE:
FALL SEMETER:
1. Laboratory Safety and Equipment (L.O. 1.3, 1.4) (S.P. 1.1, 1.4, 2.2, 5.1)
Students will:
- read and understand MSDS
- demonstrate safe laboratory practices
- demonstrate safe data analysis skills
- demonstrate correct lab reporting
Group and Report Size: Individual
2. Eight Solution Problem (L.O. 2.15, 3.1, 3.3, 3.4) (S.P. 3.3, 4.3, 5.1, 6.1)
Students will:
- use chemical and physical properties to identify compounds
Group and Report Size: Groups of Two
3. Ten Solution Problem (L.O. 2.15, 3.1, 3.3, 3.4) (S.P. 3.3, 4.3, 5.1, 6.1)
Inquiry Lab
Students will:
- use chemical and physical properties to identify compounds
Group and Report Size: Groups of Two
4. Determination of the Formula of a Hydrate (L.O. 3.5) (S.P. 2.1, 2.2, 4.3, 5.3, 6.1, 6.4)
Students will:
- use gravimetric techniques to determine the formula for a hydrate by
heating to constant mass
- use stoichiometric analysis to determine the empirical formula
Group and Report Size: Groups of Two
5. Paper Chromotography for Ion Determination (L.O. 2.7, 2.8, 2.10) (S.P. 1.4, 3.3, 5.2, 7.2)
Student will:
- use chemical and physical aspects of ions to calculate the rf values for known
and unknowns
- use the rf values and color spots to identify unknown ions based on movement in a
solvent
Group and Report Size: Groups of Two
Page 4 of 23
6. Forensic Chemistry of Empirical Formulas (L.O. 5.1, 6.4) (S.P. 2.1, 2.2, 4.2, 5.1, 5.3, 6.4, 6.5)
Inquiry Lab
Students will:
- develop a procedure using empirical formulas to identify a series of unknown
substances
- use percent composition data to determine the empirical formulas of
compounds and then use these formulas to solve a complex problem
Group and Report Size: Individual, Class Data Combined
7. Determination of Avagadro’s Number (L.O. 3.6) (S.P. 2.2,4.3, 6.1)
Students will:
- collect data to determine Avagadro’s Number based on molecular volume
Group and Report Size: Groups of Two
8. Titration of a Monoprotic Acid (L.O. 1.20, 6.13, 6.18, 6.20) (S.P. 1.4, 2.3, 3.3, 4.3, 5.1, 5.3,
6.4, 7.1)
Students will:
- collect and plot data using electronic systems
- use constructed curves to calculate values for the unknown acid
Group and Report Size: Groups of Two
9. Titration of Unknown Acids Using Indicators (L.O. 1.20, 6.13, 6.18, 6.20) (S.P. 1.4, 2.2, 3.3,
4.1, 4.2, 4.3, 5.1, 6.1)
Inquiry Lab
Students. Will:
- develop a set of procedures to identify the unknown acid molarities using titration
- use colorimetric titrations to determine molarity of unknown acids
Group and Report Size: Individual
10. A Collection of Reactions (L.O. 1.5, 2.2, 4.3, 5.1, 6.4) (S.P. 1.5, 2.2, 4.3, 5.1, 6.4)
Students will:
- analyze data to predict reaction products
- collect data using chemical and physical methods to determine reaction type
Group and Report Size: Groups of Two
11. Exothermic And Endothermic Reactions (L.O. 3.6, 3.11, 4.6, 5.3, 5.6, 5.8) (S.P. 1.4, 2.2, 3.3,
4.3, 5.2, 6.4, 7.1)
Students will:
- use enthalpy data to identify reactions as endothermic or exothermic
- generate and evaluate enthalpy graphs
Group and Report Size: Groups of Two
12. Which Metal Will Burn the Skin (L.O. 5.7, 5.8) (S.P. 2.3, 3.3, 4.1, 4.2, 4.3, 5.3, 6.1, 6.4)
Inquiry Lab
Students will:
- develop a set of procedures to identify various metals using calorimetry
- use calorimetry to identify various metals
- make comparisons between the microscopic and macroscopic structure of
metals and their observed properties
Group and Report Size: Groups of Two
13. Periodicity (L.O. 1.10, 1.11) (S.P. 4.3, 6.1, 6.4)
Students will:
- use physical and chemical properties to predict trends used to develop the periodic
table
Page 5 of 23
Group and Report Size: Groups of Two
14. Qualitative Analysis of Common Unknown Powders (L.O. 2.15, 3.3, 3.4, 3.10) (S.P. 3.3. 4.3.
5.1. 5.2. 6.4. 7.1)
Students will:
- use chemical and physical properties including solubility, pH, gas production, and
enthalpy to identify 10 unknown white powders
Group and Report Size: Groups of Two
15. Beer-Lambert Law (L.O. 1.15, 1.16, 3.4) (S.P. 4.1, 4.2, 5.1, 5.3, 6.4, 7.1)
Inquiry Lab
Students will: - produce a set of procedures to develop a serial dilution for spectrophotometric
analysis
- use colorimeters to construct concentration curves based on serial dilutions
- use Beers Law to predict concentration and absorbance values for unknowns
Group and Report Size: Groups of Two
16. A Beer’s Law Study (L.O. 1.15, 1.16, 3.4) (S.P. 4.1, 5.1, 5.3, 6.4, 7.1)
Students will:
- use colorimeters to construct concentration curves based on serial dilutions
- use Beers Law to predict concentration and absorbance values for unknowns
Group and Report Size: Groups of Four
SPRING SEMESTER:
17. Pressure-Temperature Relationships in Gases (L.O. 3.4) (S.P. 2.2, 4.3, 5.3, 6.2)
Students Will:
- analyze the collected data to examine the relationships between pressure and
temperature in gases
Group and Report Size: Groups of Two
18. Study of the Kinetics of Reactions (L.O. 1.16, 4.1, 4.2, 4.3, 4.4, 5.1) (S.P. 2.1, 2.2, 3.3, 4.3,
5.1, 6.4)
Students will:
- collect data examining the rate of reactions
- use this data to calculate and formulate reactant orders and to develop the rate law
Group and Report Size: Groups of Two
19. Rate Law Determination of a Crystal Violet Reaction (L.O. 1.16, 4.1, 4.2, 4.3, 4.4, 5.1)
(S.P. 2.1, 2.2, 3.3, 4.3, 5.1, 6.4)
Students will:
- collect data on reaction rates graphically
- using the data, determine the reactant orders and the integrated rate law
Group and Report Size: Groups of Two
20. Changing of Equilibrium (L.O. 6.8, 6.9) (S.P. 1.4, 5.2, 6.4)
Students will:
- qualitatively observe the effects of stresses on system equilibrium
Group and Report Size: Groups of Two
21. Equilibrium and LeChatliers Principle (L.O. 6.8, 6.9) (S.P. 1.4, 5.2, 6.4)
Students will:
- qualitatively observe the effects of stresses on system equilibrium
- describe and predict equilibrium changes due to LeChatlier’s Principle
Group and Report Size: Groups of Two
Page 6 of 23
22. Determination of the ka of Indicators (L.O. 2.2, 3.7) (S.P. 2.2, 3.2, 4.3, 5.2, 5.3, 6.1, 6.4)
Students will:
- use serial dilutions and indicator color to determine the indicator ka value
- predict pH of various solutions using the ka of indicators
Group and Report Size: Groups of Two
23. A Study of the pH, Dissociation, Hydrolysis, and Buffering of Solutions (L.O. 1.17, 2.1, 3.2,
3.3, 5.10, 5.16, 6.11) (S.P. 2.2, 3.2, 4.3, 4.4, 5.1, 6.1, 6.3, 7.2)
Students will:
- collect and compare the calculated and measured pH values of different acids
- use the pH data to determine ka values for various acids
- collect data from buffered solutions and then compare measured and calculated pH of
buffered solutions
Group and Report Size: Groups of Four
24. The Solubility Product Constant of Lead (II) Iodide (L.O. 6.21, 6.22, 6.23, 6.24) (S.P. 2. 2,
4.3, 5.1, 6.1)
Students will:
- use measure and collect data used in the determination of the ksp for PbI2
- use the ksp to predict the precipitation concentrations of a PbI2 solution
Group and Report Size: Groups of Two, Class Data Combined
25. Common Ion Effect and ksp (L.O. 6.21, 6.22, 6.23, 6.24) (S.P. 2.2, 4.3, 5.3, 6.2)
Students will:
- determine the effect of an added common ion on equilibrium and compound solubility
Group and Report Size: Groups of Four
26. Reactions, Predictions, and Net Ionic Equations (L.O. 1.19, 1.2, 2.1, 3.2, 3.3, 3.4, 3.8) (S.P.
1.5, 2.2, 4.3, 5.1, 6.4)
Students will:
- use physical and chemical properties such as precipitation, gas formation, color change,
and energy release to predict product formation from reactions
- write and balance net ionic equations using experimental data
Group and Report Size: Groups of Four
27. Qualitative Analysis of Unknown Solutions “The Great Flood” (L.O. 1.14, 2.10, 2.22, 3.10)
(S.P. 4.2, 5.3, 6.1, 6.2)
Inquiry Lab
Students will:
- develop chemical and physical procedures to identify unknown solids
- use physical and chemical properties to identify unknown solids
Group and Report Size: Groups of Two.
28. Which Cell Produces the Best Voltage? (L.O. 3.12, 3.13, 5.16) (S.P. 2.2, 3.2, 4.1, 4.2, 5.1,
7.1, 7.3)
Inquiry Lab
Students will:
- develop procedures to determine which combination of electrodes produce the highest
voltage
- determine the voltage produced from a cell and its use in a circuit
Group and Report Size: Groups of Two
Page 7 of 23
29. Qualitative analysis of Cations (L.O. 2.15, 3.3, 3.4) (S.P. 1.1, 2.2, 3.3, 4.3, 5.1, 6.4)
Students will:
- use chemical and physical properties to identify a series of unknown cations
- write net ionic equations using collected data
Group and Report Size: Groups of Two
30. Laser Fluorescence and Concentration of Chlorophyll (L.O. 1.16, 1.17) (S.P. 4.2, 5.1, 6.1,
6.4, 7.1, 7.2)
Inquiry Lab
Students will:
- develop the procedures needed to use laser transmittance to determine the concentrati on
of chlorophyll in an extract
Group and Report Size: Groups of Two
31. Evaporation and Intermolecular Force Interaction (L.O. 2.1, 2.3, 2.31, 5.9) (S.P. 1.2, 3.2, 3.3,
5.1, 6.2,7.1)
Students will:
- determine the effect of molecule interaction on the rate of liquid evaporation
Group and Report Size: Groups of Two
LECTURE CLASS
Due to the 90 minute block scheduling, class lecture time is listed in hours. Exams are given
after each unit consisting of multiple choice questions, mathematical problems and short essay
free response questions. Released AP questions or modified AP questions are used in the lecture
and on the exams when possible. Laboratories are referenced in each chapter, but listed before
the lecture content as part of the laboratory course with time spent on each lab not included in the
lecture schedule.
FALL SEMESTER
Chapter 1: Foundations & Introduction (CR3a)
TOPICS COVERED
Students Will:
1. Classify a substances properties as
chemical or physical.
2. Compare the properties of
compounds, mixtures & elements.
3. Perform calculations with numbers
written in exponential notation using
significant digits and SI units using
dimensional analysis.
4. Solve problems using the mass,
volume, and density relationship.
5. Distinguish between accuracy and
precision.
6. Complete Quest Assignment:
Scientific Measurements.
7. Conduct Lab: Laboratory Safety and
Equipment Use
8. Conduct Lab: 8 Solution Unknown
Problem.
ACTIVITIES
Students will:
1. Complete Quest
Assignment:
Introduction to
Chemistry.
Page 8 of 23
1.50 Hours
BIG
IDEA
1
3
EU
EK
LO
1.A
1.B
1.E
3.C
1.A.1a-d
1.B.1a-e
1.E.1.a
1.E.2.b
3.C.1.a-d
1.1
1.17
3.10
Chapter 2 and 3: Atoms, Molecules and Moles (CR3a)
TOPICS COVERED
Students Will:
1. Compare and contrast electrons,
protons and neutrons in terms of
location, charge, relative charge and
relative mass.
2. Distinguish among atoms, molecules,
ions and isotopes.
3. Determine the atomic mass of an
element based on relative isotope
abundance data.
4. Apply the following terms to
locations on the periodic table:
groups, periods, representative
elements, transition elements, innertransition elements, metals,
nonmetals, metalloids, alkali metals,
alkaline-earth metals, halogens,
noble gases.
5. Describe the general properties of
families in the representative
elements
and of the transition elements in
general.
6. Apply concepts of the mole, gramatomic mass (molar mass), molar
volume at STP and Avogadro's
number in problem-solving for
elements and compounds.
7. Conduct Inquiry Lab: 10 Solution
Unkown Problem.
8. Conduct Lab: Determination of
Avagadro’s Number.
ACTIVITIES
Students Will:
1. Complete Quest
Assignment Moles
and Reactions.
2. Use mass
spectrophotometer
data of the relative
isotope masses and
percentages to
determine the
average mass of
elements.
(L.O. 1.14)
2.25 Hours
BIG
IDEA
1
3
EU
EK
LO
1.A
1.B
1.E
3.C
1.A.1a-d
1.B.1a-e
1.E.1.a
1.E.2.b
3.C.1
1.1
1.17
3.10
Chapter 2 and 3: Formulas and Nomenclature (CR3b)
TOPICS COVERED
ACTIVITIES
Students Will:
1. Discuss the differences between ionic
and molecular (covalent) compounds.
2. Identify and use elements and ions.
3. Write formulas and/or names for ionic
compounds, molecular (covalent)
compounds, acids and oxyacids, and
selected organic compounds including
simple alkanes, alkenes, alkynes,
alcohols and carboxylic acids
containing chains of 1-10 carbons.
4. Calculate the molar mass of a
substance; use the molar mass and
Students Will:
1. Complete Quest
Assignment
Chemical
Nomenclature.
2. Given mass
spectrophotometer
data showing
percent
compositions,
determine the
empirical and
molecular
Page 9 of 23
5.25 Hours
BIG
IDEA
1
2
5
EU
EK
LO
1.A
2.A
2.B
5.D
1.A.1.c,d
1.A.2.a-c
1.A.3.a-d
1.E.2.a
2.A.3,e,f
2.B.1.a-c
5.D.3.b
1.1
1.2
1.3
1.4
1.17
1.18
2.10
2.11
5.11
Avogadro's number to convert among
mass, moles and number of particles.
5. Determine the percent composition (by
mass) of a compound from its formula
and/or from lab data and determine
the empirical and molecular formulas.
6. Conduct Lab: Determination of the
Formula of a Hydrate.
formulas for
substances.
(L.O. 1.1, 1.2)
Chapter 22: Organic (CR3a)
TOPICS COVERED
Students Will:
1. Write condensed and expanded
structural formulas and name alkanes,
alkenes and alkynes.
2. Draw isomers (including cis-trans).
3. Describe the structure and bonding of
benzene.
4. Recognize the basic functional groups
(alcohols, carboxylic acids, esters,
ketones, aldehydes, ethers, and
amines, amino acids) , name and draw
structures for compounds containing
these groups, and describe general
characteristics of these groups.
5. Describe the structures, functions and
formation of proteins, and their role in
DNA and RNA.
6. Be familiar with the structures and
characteristics of carbohydrates and
lipids.
7. Conduct Inquiry Lab: Forensic
Chemistry of Empirical Formulas.
3.00 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment
Organic
Chemistry.
BIG
IDEA
1
2
5
EU
EK
LO
1.A
2.A
2.B
5.D
1.A.1.c,d
1.A.2.a-c
1.A.3.a-d
1.E.2.a
2.A.3,e,f
2.B.1.a-c
5.D.3.b
1.1
1.2
1.3
1.4
1.17
1.18
2.10
2.11
5.11
Chapter 3: Chemical Equations and Stoichiometry (CR3c)
TOPICS COVERED
Students Will:
1. Write and balance chemical equations
for these types of reactions:
(a) Redox, composition, decomposition,
single and double displacement.
(b) Combustion of hydrocarbons.
(c) Precipitation reactions.
(d) Common acid-base reactions.
(e) Reaction of acidic and basic
anhydrides.
2. Calculate the masses of reactants and
products using chemical equations
(stoichiometric calculations) including
the use of limiting reagents.
3. Calculate theoretical and percentage
yields of reactions.
ACTIVITIES
Students Will:
1. Complete Quest
Assignment
Chemical
Reactions.
Page 10 of 23
6.00 Hours
BIG
IDEA
1
2
3
5
6
EU
EK
LO
1.A
1.D
1.E
2.A
3.A
3.B
3.C
6.C
1.A.1.a-d
1.A.2.a-c
1.A.3.a-d
1.D.2.a-c
1.E.1.a-c
1.E.2.a,c-f
2.A.3.a, h-j
3.A.1.a-d
3.A.2.a
3.A.2.c
3.B.1.a
3.B.3.a-d
3.C.1.d
6.C.3.d
1.1
1.2
1.3
1.4
1.14
1.17
1.18
1.19
2.8
2.9
2.14
3.1
3.2
3.3
3.4
3.5
4. Perform percentage and empirical
formula calculations using reaction
data.
5. Identify and write net ionic equations
for reactions.
6. Conduct Lab: A Collection of
Reactions.
7. Conduct Lab: Qualitative Analysis of
Common Unknown Powders.
3.6
3.8
3.9
3.10
Chapter 4 and 11: Solutions and Reactions in Aqueous Solutions (CR3c)
TOPICS COVERED
ACTIVITIES
Students Will:
1. Describe the nature of aqueous
solutions including the action of water
as a solvent and characterize strong
and weak electrolytes.
2. Write equations for dissolution of
electrolytes and nonelectrolytes and
write net ionic equations for reactions
in aqueous solutions.
3. Use the concepts of molecular
structure, pressure and temperature to
explain solubility of a solute in a
given solvent.
4. Apply Le Chatelier's Principle to the
factors affecting solubility.
5. Explain the effects of colligative
properties on the properties of
solutions.
6. Determine the f.p. or b.p. of a
nonelectrolytic solution or calculate
the molar mass of a nonvolatile solute
from f.p. or b.p. data.
7. Describe the applications of osmosis
and osmotic pressure; relate osmotic
pressure mathematically to solution
concentration.
8. Compare the colligative properties of
electrolytes to those of
nonelectrolytes.
9. Characterize and give examples of
colloidal dispersions.
10. Identify common strong and weak
acids and bases; write their
dissociation equations.
11. Determine the solubility of ionic
compounds from general solubility
rules.
12. Describe the preparation of and
calculate the molarity of a specified
solution.
13. Calculate mass of solute, volume of
Students Will:
1. Complete Quest
Assignment
Solution Basics.
2. Complete Quest
Assignment Redox
Reactions.
3. Complete Quest
Assignment :
Solution
Properties.
4. Balance redox
reactions using the
half reaction
procedure.
(L.O. 3.8, 3.9)
5. Balance by
oxidation number
change. (L.O. 3.8,
3.9)
6. Will investigate the
major components
of acid rain
writing the
reactions that
occur between
pollution and
compounds in
compounds
naturally found in
the environment
(water, oxygen,
carbon dioxide,
etc.)
(L.O.3.2) [CR4]
Page 11 of 23
BIG
IDEA
1
2
3
6
13.50 Hours
EU
EK
LO
1.E
2.A
2.D
3.A
3.B
6.C
1.E.1.a-c
1.E.2.a-f
2.A.1.c,e
2.A.3.a-j
2.B.2.b
2.B.3.a,b
2.D.1.a.1-5
3.A.1.a-d
3.B.2.b
3.B.3.a-e
6.C.1.a-d
6.C.3.d
1.16
1.17
1.18
1.20
2.1
2.2
2.3
2.7
2.8
2.9
2.10
2.13
2.15
2.16
2.24
3.2
3.7
3.8
3.9
6.11
6.12
6.13
6.15
solution or concentration of solution
using molarity as the concentration
term.
14. Convert among concentration terms
for solutions: molarity, molality, mass
percent, mole fraction.
15. Interpret and apply qualitative
concentration terms: saturated,
supersaturated, unsaturated, miscible,
immiscible.
16. Perform dilution calculations.
17. Perform stoichiometric calculations
for solutions using molarity.
18. For metathesis reactions:
(a) Predict the products of these
reactions, identifying the reactants
& products by phases.
(b) Perform stoichiometric calculations
for these reactions.
(c) Perform calculations involved in acidbase volumetric analysis.
(d) Perform calculations of chemical
analysis of precipitation reactions.
19. Describe the process and uses of
titration
20. Assign oxidation numbers to various
the elements in various species and
identify the oxidized and reduced
substances.
21. Balance redox reactions in acidic and
basic solutions.
22. Perform calculations associated with
redox titrations.
23. Conduct Lab: Paper Chromotography
for Ion Determination.
24. Conduct Lab: Acid/Base Titration of
a Monoprotic Acid.
25. Conduct Inquiry Lab: Titration of
Unknown Acids Using Indicators.
Chapter 18: Electrochemistry (CR3e)
TOPICS COVERED
Students Will:
1. Assign oxidation numbers to each
element in a compound or ion and
identify oxidizing and reducing
agents.
2. Write and balance net ionic equations
for redox reactions.
3. Solve titration problems for redox
reactions, using molarity.
4. Distinguish between galvanic and
electrolytic cells.
5. For voltaic, electrolytic or galvanic
6.00 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment:
Electrochemistry.
2. Evaluate and
construct voltaic
cell diagrams and
determine cell
potential using
redox equations.
(L.O. 3.12, 3.13)
Page 12 of 23
BIG
IDEA
3
5
6
EU
EK
LO
3.A
3.B
3.C
5.E
6.A
3.A.1.a
3.B.3.a,c,d
3.C.3.a-f
5.E.4.a
6.A.1.b
3.2
3.8
3.12
3.13
5.15
6.1
cells:
(a) Diagram and label parts of the cell,
including electron flow.
(b) Write half-reactions for processes at
the electrodes.
(c) Write the balanced equation and the
line notation for the cell.
(d) Determine the anode and cathode
from reaction data.
6. Use standard reduction potentials to
compare strengths of oxidizing and
reducing agents, to calculate cell
potential; and to predict spontaneity
of a redox reaction.
7. Use the Nernst Equation to calculate
EMF at non-standard conditions.
8. Relate cell potential to free energy and
equilibrium values.
9. Apply Faraday's Law to electrolytic
cells in calculating amount of
products formed, time or current
required or energy used.
10. Explain the electrochemical nature of
lead storage batteries, corrosion.
12. Relate voltaic cells and Nernst to free
energy and equilibrium.
13. Conduct Inquiry Lab: Which Cell
Produces The Best Voltage?
Chapter 6: Thermochemistry (CR3e)
6.00 Hours
TOPICS COVERED
ACTIVITIES
Students Will:
1. Describe the energy flow between a
system and its surroundings.
2. Explain the significance of the first
law of thermodynamics.
3. Distinguish among heat, temperature,
work, kinetic and potential energies.
4. Use calorimetric data to determine
the energy changes that occur.
5. Describe and discuss energy and the
relationships between phases.
6. Use Hess's law to calculate the
enthalpy change.
7. Write and solve equations to define
enthalpies of formation.
8. Conduct Lab: Exothermic and
Endothermic Reactions.
9. Conduct Inquiry Lab: Which Metal
Will Burn The Skin?
Students Will:
1. Complete Quest
assignment on
thermochemistry.
2. Given sets of
reaction pathways,
determine the total
enthalpy change for
a reaction.
(L.O. 5.6)
Page 13 of 23
BIG
IDEA
3
5
EU
EK
LO
3.C
5.A
5.B
5.C
5.E
3.C.2.a-d
5.A.2.a-f
5.B.1.a-c
5.B.2.a,b
5.B.3.a,b,e,f
5.B.4.a-c
5.C.2.c,f,g
5.E.2.a
3.11
5.3
5.4
5.5
5.6
5.7
5.13
OBJECTIVES: Chapter 17: Thermodynamics (CR3e)
TOPICS COVERED
ACTIVITIES
Students Will:
1. Discuss First Law of
Thermodynamics.
2. Define entropy in terms of positional
probability.
3. Predict and relate the signs of H
and S to "favored" direction of a
reaction.
4. Describe and evaluate the Second
and Third Laws of
Thermodynamics.
5. Apply the relationship between S,
H to the surroundings.
6. Calculate S for reactions or phase
changes.
7. Use the Gibbs-Helmholtz equation to
calculate the free energy for a
reaction and relate G, H, and S
to reaction spontaneity.
8. Relate thermodynamic values of
enthalpy, entropy, and free energy to
equilibrium and electrochemistry
both qualitatively and quantitatively.
Students Will:
1. Complete Quest
Assignment:
Thermodynamics.
2. Given a data sets,
determine if the
situation is
thermodynamically
favored or not by
looking at entropy,
enthalpy, and
Gibb’s Free Energy.
(L.O. 5.13)
7.50 Hours
BIG
IDEA
2
5
6
EU
EK
LO
2.B
5.A
5.C
6.D
2.B.3.b
5.A.2.a,b,c,e
5.C.2.d,e
5.E.1.a,b,c
5.E.2.b-f
5.E.3.a-c
5.E.4.a-c
5.E.5.a,b
6.D.1.a-d
2.15
5.3
5.12
5.13
5.14
5.15
5.16
5.17
5.18
6.25
Chapter 7: Quantum Mechanics, Atomic Structure and Periodicity (CR3a)
TOPICS COVERED
ACTIVITIES
Students Will:
1. Describe and discuss atomic models and
their formation.
2. Describe, relate and quantify the
electromagnetic spectrum sections of
the spectrum, relative frequencies,
wavelengths and energies of the
sections.
3. Use photoelectron spectrophotometry
data to explain and evaluate electron
location.
4. Describe Planck's concept of
quantitized energy.
5. Calculate the energy of a photon using
the relationship A = hv.
6. State the significance of the de Broglie
relationship, and use this
relationship in calculations.
7. Relate Bohr's model of the atom to the
quantum theory.
8. Calculate the energy difference
resulting from the change in electron
Students Will:
1. Complete Quest
Assignment:
Quantum Theory.
2. Complete Quest
Assignment:
Periodic Trends.
3. Given sample trend
data, predict and
formulate a
simulated periodic
table.
(L.O. 1.9)
Page 14 of 23
BIG
IDEA
1
4
5
10.50 Hours
EU
EK
LO
1.B
1.C
1.D
4.A
5.E
1.B.1.a-d
1.B.2.a-d
1.C.1.a-d
1.C.2
1.D.1.a,b
1.D.3.a,b
4.A.1.b
5.E.4.b.1
1.5
1.6
1.7
1.8
1.9
1.10
1.11
1.12
1.13
1.15
Need
4.a.1
5.e.4
levels of an electron; calculate the
ionization energy for an electron.
9. Describe the contributions of
Heisenberg and Schrodinger to the
wave mechanical model concept of
the atom.
10. State the meaning and possible values
of the quantum numbers assigning
them to a given sublevel or orbital.
11. Describe a given sublevel, orbital or
electron in quantum number terms and
use Hund's Rule to assign an electron
configuration for a given element or
ion.
12. Construct the orbital diagram of an
element.
13. Identify paramagnetism and
diamagnetism through electronic
structure.
14. Describe how effective nuclear
charge varies with position on the
periodic table.
15. Use Coulomb’s Law to explain
periodic patterns and electron
locations.
16. Using the electron configurations and
the concept of effective nuclear
charge, interpret the trends within the
periodic table for the following
properties: atomic and ionic radii,
ionization energy, electron affinity.
17. Conduct Lab: Beers Law: A Study.
18. Conduct Inquiry Lab: Beer-Lambert
Law.
Chapter 19: Nuclear Chemistry (CR3a)
TOPICS COVERED
Students Will:
1. For alpha, beta and gamma radiation
describe each type of radiation,
mass, charge, relative penetrating
power, symbol and its emission
effect on atomic number and atomic
mass.
2. Write and balance nuclear equations
for emission of each type of
radioactive decay and nuclear
transformations.
3. Generally describe the biological
effects of radiation and the units in
which it is measured.
4. Predict the type of radioactive decay
for a given isotope, using n o/p+
ratio as a guide.
2.25 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment:
Nuclear Chemistry.
2. Simulate half-life
using candy and
generate graphs
showing the rate of
decay.
(L.O. 4.3)
3. Investigate the
potential problems
of using nuclear
materials on the
environment and
humans.
(L.O. 4.3) [CR4]
Page 15 of 23
BIG
IDEA
1
4
EU
EK
LO
1.D
4.A
1.D.2.a-c
4.A.1.a
4.A.3.e
1.14
4.1
4.3
5. Use the first order rate law to relate
the amount of a radioactive species
to elapsed time.
6. Given a table of nuclear masses,
calculate mass for a nuclear reaction
and relate it to energy change.
7. Generally describe the functioning,
reactions, and positive and negative
aspects of fission, and fusion
reactors.
8. Describe the experiments used to
identify radioactive particles.
SPRING SEMESTER
Chapter 5: Gases (CR3f)
TOPICS COVERED
Students Will:
1. Define and convert pressure units.
2. Describe Boyle's, Charles', GayLussac's, Avogadro's and Combined
Laws concerning gases & perform
calculations involving these laws.
3. Use the Ideal Gas Equation to
determine the effects of a change in
one or more variables on others.
4. Calculate the density or molar mass
of a gas and solve stoichiometric
calculations at standard and nonstandard conditions.
4. Use Dalton's Law to relate partial
pressure and total pressures of a
mixture of gases; relate partial
pressures to mole fractions.
5. Use the basic postulates of the
Kinetic Molecular Theory to explain
the gas laws and properties of gases.
6. Use Graham's Law to relate the molar
masses of gases to their rates or
times of effusion.
7. Describe how real gases deviate from
ideal behavior; show how van der
Waals's equation allows for real
conditions (qualitative only).
8. Graphically determine the
relationship between two variables
and write equations relating the
variables.
9. Describe and discuss Le Chatlier’s
Principle and gas phase equilibrium.
10. Conduct Lab: Pressure-Temperature
Relationships in Gases.
3.75 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment: Gas
Behavior.
Page 16 of 23
BIG
IDEA
1
2
3
5
EU
EK
LO
1.A
2.A
2.B
3.A
5.A
1.A.2.a-c
1.A.3.a-d
2.A.2.a-g
2.B.2.a,d
2.B.3.c,d
3.A.2.a.2
3.A.2.b
5.A.1.a,b
5.A.1.c
1.3
1.4
2.4
2.5
2.6
2.12
2.15
3.4
5.2
Chapter 12: Kinetics (CR3d)
5.25 Hours
TOPICS COVERED
ACTIVITIES
Students Will:
1. Describe the collision theory and the
requirements for effective collisions.
2. Sketch and/or interpret graphs of
endothermic and exothermic
reactions, identifying the activation
energy, enthalpies, and the reaction
course with and without catalyst.
3. Provide a molecular explanation for
the factors that affect the rate of a
reaction: nature of reactants, surface
area, concentration, physical state
and catalysis.
4. Write the rate equations for a reaction
in terms of each reactant or
product.(Rate = [conc]/ time)
5. From experimental data, graphically
determine the rate law for a reaction.
6. Determine a zero, first or second
order reaction from graphical
analysis of concentration vs. time
plots.
7. Determine the rate expression,
reaction order and rate constant from
rate and concentration data.
8. For a first-order reaction, determine
the concentration of reactant after a
given time and the time required for
the concentration to drop by a given
amount.
9. Determine whether a proposed
mechanism for a reaction is
consistent with the observed rate
expression, or suggest a mechanism
that is consistent with the observed
rate expression using Hess Law.
10. Conduct Lab: Study of the Kinetics
of Reactions.
11. Conduct Lab: Rate Law
Determination of Crystal Violet.
Students Will:
1. Complete Quest
Assignment:
Reaction Kinetics.
BIG
IDEA
4
EU
EK
LO
4.A
4.B
4.C
4.D
4.A.1.a,c
4.A.2.a-c
4.A.3.a-e
4.B.1.a,b
4.B.2.a-d
4.B.3.a-c
4.C.1.a-c
4.C.2.a
4.C.3.a,b
4.D.1.a,b
4.D.2.a-c
4.1
4.2
4.3
4.4
4.5
4.6
4.7
4.8
4.9
Chapter 13: General Concepts of Equilibrium (CR3f)
TOPICS COVERED
Students Will:
1. Discuss how equilibrium is
established.
2. Write the equilibrium expression for a
given equilibrium system in terms of
ACTIVITIES
Students Will:
1. Complete Quest
Assignment:
Chemical
Equilibria.
Page 17 of 23
5.25 Hours
BIG
IDEA
6
EU
EK
LO
6.A
6.B
6.A.1.a,b
6.A.2.a-c
6.A.3.a-f
6.A.4.a,b
6.B.1.a,b
6.1
6.2
6.3
6.4
6.5
concentrations or pressures.
3. Calculate values for the equilibrium
constant.
4. Manipulate the equilibrium constant
expressions and values when changing
stoichiometric coefficients of the
equation, reversing the equation and
when adding equations.
5. Convert between Kc and Kp and
determine the equilibrium
concentrations for the species.
6. Use the reaction quotient, Q, to
determine the initial direction of a
reaction needed to establish
equilibrium.
7. Predict the changes in equilibrium that
will occur when various stresses
are placed on the system (Le Chatlier's
Principle): concentration change,
temperature change, pressure change
and addition of a catalyst.
8. Predict the changes in the value of the
equilibrium constant that would
occur as the temperature changes .
9. Conduct Lab: Changing of
Equilibrium.
10. Conduct Lab:Equilibrium and
LeChatlier’s Principle.
2. Given a chemical
data set showing
stresses, evaluate
and determine the
changes and shifts
in equilibrium.
(L.O. 6.8)
6.B.2.a,b
Chapter 14: Acids and Bases (CR3f)
6.6
6.7
6.8
6.9
6.10
5.25 Hours
TOPICS COVERED
ACTIVITIES
Students Will:
1. Identify strong and weak acids and
bases and write dissociation equations
for each.
2. Write and analyze the Kw expression
for water.
3. Calculate [H+], [OH-], pH or pOH for
strong acids or bases from data or
graphs.
4. Write empirical and net ionic
equations for acid-base reactions.
5. Identify acid or base anhydrides; write
equations for formation of acids
or bases from anhydrides.
6. Describe and evaluate the three
acid/base theories.
7. Identify Bronsted-Lowry acids and
bases and the conjugate pairs;
compare strengths of the B-L acids or
bases.
8. Predict the direction of equilibrium
from a knowledge of the strength of
the acid-base conjugate pair in water.
Students Will:
1. Complete Quest
Assignment: Acids
and Bases.
2. Given titration
data, determine pH
and chemical
molarities at
various points
along the curve.
(L.O. 6.13, 6.15)
Page 18 of 23
BIG
IDEA
2
3
6
EU
EK
LO
2.B
3.B
6.A
6.C
2.B.2.a,d
3.B.2.a
3.B.2.b.1-3
6.A.1.a,b
6.C.1.a-o
2.1
2.2
3.7
6.1
6.11
6.12
6.13
6.14
6.15
6.16
9. Predict whether a given salt will
hydrolyze to form an acidic, basic or
neutral solution and write equations
for hydrolysis of salts or ions.
10. Calculate the pH of a solution of a
salt which hydrolyzes in water to form
an acidic or basic solution.
11. Relate strengths of weak acids or
bases to Ka, Kb or pKa.
12. Write the equilibrium expression for
a weak acid or base and interpret this
expression to obtain pH, ion
concentrations, Ka or Kb, %
dissociation, when given appropriate
data.
13. Calculate the concentration of each
species and pH in a weak polyprotic
acid solution.
14. Conduct Lab: Determination of the
ka of Indicators.
Chapter 15: Acid-Base Reactions (CR3f)
TOPICS COVERED
Students Will:
1. Describe and evaluate the acid base
theories.
2. Perform stoichiometric calculations
for acid-base reactions.
3. Predict the direction of an acid-base
reaction.
4. Write a net ionic equation for a given
acid/base reaction.
5. Determine pH at intervals and at the
equivalence point during a strong
acid-strong base acid-base titration.
6. Determine the appropriate indicator
for an acid-base reaction and
describe how chemical indicator
changes colors, based on pH
changes.
7. Graph and interpret titration curves
for strong acid-strong base, strong
acid-weak base, weak acid-strong
base titrations.
8. Graphically determine pKa for a
weak acid from a titration curve.
9. Calculate the ion concentrations and
pH of aqueous salt solutions
(hydrolysis problems).
10. Describe the action of buffers.
11. Given the composition of a buffer
system, determine its pH before and
after the addition of known amounts
of strong acid or base.
4.50 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment:
Buffers and
Hydrolysis.
2. Given sets of acids
and bases,
determine and
select the best
indicator to use.
(L.O. 6.13, 6.15)
Page 19 of 23
BIG
IDEA
1
3
6
EU
EK
LO
1.E
2.B
3.A
3.B
6.A
6.C
1.E.2.f
2.B.2.a,d
3.A.2.c
3.B.2.b.1-3
6.A.1.a,b
6.A.2.b
6.A.3.a-d
6.C.1.a-o
6.C.2.a-d
1.20
2.1
2.2
3.3
3.7
6.1
6.12
6.13
6.14
6.15
6.16
6.17
6.18
6.19
6.20
12. Determine the proportions in which
a weak acid and its conjugate base
should be mixed to give a specified
pH buffer.
13. Conduct Lab: Study of the pH,
Dissociation, Hydrolysis, and
Buffering of Solutions.
Chapter 16: Solubility Equilibrium (CR3f)
TOPICS COVERED
ACTIVITIES
Students Will:
1. Write the Ksp expression for a salt in
water.
2. Calculate the solubility product of a
salt given its solubility and predict
the relative solubilities of compounds
from Ksp values.
3. Calculate the molar solubility for a
salt and use Qsp to determine
whether a precipitate will form upon
combination of two solutions.
4. Determine the molar solubility for a
salt when placed in a solution having
a common ion.
5. Given appropriate data, calculate the
ion concentration required to begin
precipitation.
8. Describe the use of selective
precipitation to separate a mixture of
ions in solution.
9. Show how complex ion formation can
increase the solubility of a salt.
10. Conduct Lab: Solubility Product
Constant of Lead (II) Iodide.
11. Conduct Lab: Common Ion Effect
and ksp.
Students Will:
1. Complete Quest
Assignment:
Solubility
Equilibria.
2. Given salt solubility
data, evaluate and
predict the effects of
common ions on
precipitation.
(L.O. 6.23)
6.75 Hours
BIG
IDEA
6
EU
EK
LO
6.A
6.C
6.A.4.a,b
6.C.3.a,b,c
6.C.3.e,f
6.21
6.22
6.23
6.24
Chapter 8 and 9: Bonding (CR3b)
TOPICS COVERED
Students Will:
1. Compare the general nature of ionic
and covalent bonds.
2. Use Coulomb’s Law to evaluate and
explain bond strength and size.
3. Relate the enthalpy of dissociation of
an ionic bond to the bond strength.
4. Write Lewis dot structure for
molecules or polyatomic ions,
including species that are exceptions
to the octet rule.
5. Predict and write dot structure for
resonance structures.
7.50 Hours
ACTIVITIES
Students Will:
1. Complete Quest
Assignment: Ionic
and Covalent
Bonding.
2. Complete Quest
Assignment:
Molecular
Geometry.
3. Given molecular
model sets,
determine the
geometry,
Page 20 of 23
BIG
IDEA
1
2
5
EU
EK
LO
1.B
1.C
2.C
2.D
5.C
1.B.2.a-d
1.C.1.c
2.C.1.a-f
2.C.2.b
2.C.4.a-1
2.D.1.b
5.C.1a-e
5.C.2.a,b
1.11
1.15
2.1
2.17
2.18
2.19
2.21
2.22
2.23
2.24
5.1
5.8
6. Relate electronegativity values to
bond polarity.
7. Compare bond distance and bond
energy for single or multiple bonds.
8. Use bond energies to calculate
enthalpies of formation for
chemicals.
9. Compare oxidation numbers and
formal charges in molecules and
ions.
10. Use the formal charges to determine
the most reasonable resonance
structure.
11. Use the VSEPR Model to predict
and identify molecular geometry.
12. Determine the dipole moment
vectors for individual bonds and for
an entire molecule; determine
molecular polarity.
13. Correlate VSEPR structures to the
predicted hybridization of orbitals.
14. Contrast the formation and
characteristics of sigma and pi bonds,
and predict the number of sigma and
pi bonds in a species.
15. Conduct Lab: Reactions,
predictions, and Net Ionic Equations.
16. Conduct Inquiry Lab: Qualitative
Analysis of Unknown Solution.
hybridization, and
polarity of selected
compounds
(L.O. 2.21)
Chapter 10: Solids and Liquids (CR3b)
6.00 Hours
TOPICS COVERED
ACTIVITIES
Students Will:
1. Describe and compare the
intermolecular forces (IMF) : dipoledipole, hydrogen bonding, London
(dispersion) forces.
2. Describe the effects that
intermolecular forces have on the
properties of liquids and solids ( e.g.,
effects on m.p. and b.p., vapor
pressure, state at room temp.,
viscosity, surface tension, changes of
phase, solubility).
3. Relate the magnitude of vapor
pressure to temperature, IM forces.
4. Describe the processes of
evaporation, condensation,
sublimation and fusion on a particle
level. Explain the relationship of
boiling point to vapor pressure.
5. Explain the significance of critical
temperature and pressure.
6. Interpret heating and cooling curves.
Students Will:
1. Complete Quest
Assignment: Liquids
and Solids.
Page 21 of 23
BIG
IDEA
1
2
5
EU
EK
LO
1.C
2.A
2.B
2.C
2.D
5.B
5.D
1.C.1.d
2.A.1.a-e
2.B.2.a-d
2.B.3.a,b
2.C.2.a
2.C.2.b.2
2.C.3.a-d
2.D.1.a.1-3
2.D.1.a.5
2.D.1.b
2.D.2.a,b
2.D.3.a-c
2.D.4.a,b
5.B.3.c,d
5.D.2.a,b
5.D.3.a,b
1.11
2.3
2.13
2.14
2.15
2.16
2.20
2.23
2.24
2.25
2.26
2.27
2.28
2.29
2.30
2.31
2.32
5.6
5.9
5.10
5.11
7. Calculate energy changes during
phase changes.
8. Describe the following properties of
liquids: surface tension, capillary
action and viscosity.
9. Apply the concepts of unit cells and
crystal lattices for solids to
calculations involving atomic radii,
volume, density or identity.
10. Distinguish among ionic, molecular,
network covalent and metallic solids
with regard to particle structure,
physical properties and inter- and
intra-molecular forces.
11. Describe the unique characteristics
of water due to hydrogen bonding.
12. Conduct Lab: Qualitative Analysis
of Cations.
13. Inquiry Lab: Laser Fluorescence and
Concentration of Chlorophyll
14. Evaporation and Intermolecular
Force Interaction.
TEXTBOOK, LABORATORY MANUAL, AND STUDY GUIDES
Zumdahl, S.S. and S.A. Zumdahl. Chemistry, 9th ed. AP, Brooks/Cole, Cengage Learning, 2013.
[CR1]
Bauer, R.D., et. al. Laboratory Inquiry in Chemistry, Brooks/Cole, 2005
Brown, T.L., et. al. Chemistry The Central Science 12th AP ed., Pearson Prentice Hall, Inc.,
2012.
Chang, R. and K.A. Goldsby. Chemistry, 11th AP ed., McGraw-Hill Co., Inc., 2013.
College Board Released Materials and Problems.
Conference for the Advancement of Science Teachers (CAST), Handouts, 1990-1995
DeCoste, D.J. Inquiry Based Learning Guide For Zumdahl and Zumdahl’s Chemistry, 8 th ed.,
Brooks/Cole, Cengage Learning, 2010.
Ehrenkranz, D. and J.J. Mauch. Chemistry in Microscale, Kendall/Hunt Pub. Co, 1990.
Flinn Advanced Placement Laboratory Kits. Flinn Scientific Catalog. Flinn Scientific, Inc.,
2013.
Goodman, and Petrucci. The Solubility product of Lead (II) Iodide. J. Chem. Ed., 42:104, 1065.
Hague, G.R.,Jr. and J.D. Smith. The Ulitmate Chemical Equations Handbook., Flinn Scientific,
Inc., 2001.
Holmquist, D.D., et. al. Chemistry With Calculators, Vernier Software Technology, 2000.
Jones, C., 1995 Personal Materials.
Page 22 of 23
Kotz, J.C., et al. Chemistry and Chemical Reactivity, 8th ed., Brooks/Cole Cengage Learning,
2011
Lab-Aids Laboratory Kits. Lab-Aids, Inc., 2013.
Milo, F.R., N.W.G. Debye, and C. Metz. Experiments in General Chemistry, Saunders
Publishing Co., 1991.
Randall, J. Advanced Chemistry With Vernier, Vernier Software and Technology, 2006.
Russo, T. MicroChemistry, Volume 2., Kentec Educational Corporation, 1991.
Science Kit Advanced Placement Laboratory Kit. Science Kit Cataloq. Science Kit, Inc.. 2013.
Vonderbrink S.A., Laboratory for Advanced Placement Chemistry, Flinn Scientific, Inc., 1995.
Waterman, E.L. AP Chemistry, AP Prep Series, Prentice Hall, 2012.
Weiner, S.A. and E.I. Peters. Introduction to Chemical Principles, A Laboratory Approach, 3rd
ed., Saunders College Publishing Co., 1986.
Whitten, K.W., et al. General Chemistry, 13 th ed., Brooks/Cole Cengage Learning, 2013.
Page 23 of 23