TURNER Unit Planner 2015
advertisement

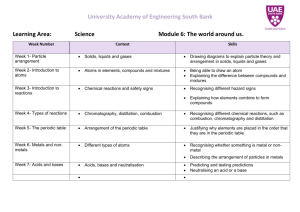
UNIT PLANNER Subject: Term/Year: Unit Title: Assessment: Key Resource: SCIENCE 2 / 2015 CHEMISTRY E.E.I. Click here to enter text. Year Level 10 Key ideas from curriculum documents Science Understanding Different types of chemical reactions are used to produce a range of products and can occur at different rates (ACSSU187) investigating how chemistry can be used to produce a range of useful substances such as fuels, metals and pharmaceuticals predicting the products of different types of simple chemical reactions using word or symbol equations to represent chemical reactions investigating the effect of a range of factors, such as temperature and catalysts, on the rate of chemical reactions The atomic structure and properties of elements are used to organise them in the Periodic Table (ACSSU186) recognising that elements in the same group of the periodic table have similar properties describing the structure of atoms in terms of electron shells explaining how the electronic structure of an atom determines its position in the periodic table and its properties investigating the chemical activity of metals Science as a Human Endeavour Scientific understanding, including models and theories, are contestable and are refined over time through a process of review by the scientific community (ACSHE191) investigating the development of the periodic table and how this was dependent on experimental evidence at the time Science Inquiry Skills Questioning and predicting Planning and conducting Processing and analysing data and information Evaluating Communicating. KEY REQUIREMENTS Literacy • Comprehending texts through listening, reading and viewing • Composing texts through speaking, writing and creating • Text knowledge • Grammar knowledge • Word knowledge • Visual knowledge Numeracy Recognising and using patterns and relationships Interpreting statistical information ICT capability • Investigating with ICT Critical and creative thinking • Inquiring - identifying, exploring and organising information and ideas • Generating ideas, possibilities and actions • Reflecting on thinking and processes • Analysing, synthesising and evaluating reasoning and procedures DIFFERENTIATION Students requiring support can… The learning experiences within this unit can be differentiated by increasing: • the frequency of exposure for some students • the intensity of teaching by adjusting the group size • the duration needed to complete tasks and assessment Students requiring extension can… For guided and/or independent practice tasks: • student groupings will offer tasks with a range of complexities to cater for individual learning needs • rotational groupings that allow for more or less scaffolding of student learning Document1 Term 2 Week 1 2015 Lesson 1 Lesson 2 Lesson 3 1. PERIODIC TABLE and REACTIVITY Review atoms, (1ST 20) elements and Periodic Table , PowerPoint Presentation – Chemistry Review Reactions of Metals Compare the reactivity and predicting the products of common metals with acid, oxygen and water Alkali metals in water Experiment – Exploring the reactions of metals Patterns in the first 20 Elements Identify patterns and trends in the first 20 elements, Mendeleev Activity – website game 2 3 4 5 6 2. Chemical Formulas Ion Formulas Table 3. Equations Writing Chemical Equations Worksheet /Writing Balanced Equations Worksheet 4. Bohr Model OF THE ATOM Introduce and explore the Bohr model Introduce concept of energy shells and electron arrangement diagrams, Flame test Consolidate electron arrangement through addition of electron arrangement diagrams to element PowerPoint – Electron Arrangement Exploring the Periodic Table Relationship between electron arrangement and element location on the Periodic Table ,PowerPoint-Periodic Table Using the Periodic Table Relate patterns in electron arrangement to Periodic Table and apply these to predict the properties of elements including unfamiliar elements. Using the periodic table worksheet Relating Reactivity to Electron Arrangement Represent formation of ions using electron dot diagrams Introduce the concept of ionic bonding Predict compound formulas for simple ionic compounds using electron arrangement diagrams Learning Object Elements/You tube video- Ionic Bond 5. IONIC COMPOUNDS Understand and apply conventions for writing formulas and naming ionic compounds PowerPoint Ionic Compounds/BBC Bitesize Ionic Activity Properties of Ionic Compounds Investigate the properties of ionic compounds including solubility and electrical conductivity of solutions Explore precipitate reactions, introducing and applying solubility rules to predict precipitate formation Youtube video-Ionic Compound 6. IONIC COMPOUNDS Use created solubility table to predict precipitates and identify unknowns Youtube- Dissociation of NaCl Flame Tests Template Solubility Experiment Predicting Precipitate Solubility Rules Worksheet/Teacher Notes/Research Assignment Investigating reaction rates Experiments to investigate reaction rates Factors that influence reaction rate, temp, catalyst, surface area, concentration Continue experiments on Reaction Rates – discuss results summarises reaction rates 9 7. Writing an EEI. Extended Experimental investigation handed out How to write a scientific method Students write method + apparatus over weekend individually Research Generate investigation question and hypothesis Method writing Method checked by teacher prior student’s conducting experiment Perform Experiment Perform Experiment Discuss experimental results Results Draft Checked by teacher Students write report Students write report EXTENDED EXPERIMENTAL INVESTIGATION REPORT 10 Catch up and finalise assessment 7 8 Literacy Focus Compound Molecule Solubility Nucleus Numeracy Focus ICT lesson Creative Thinking Atomic Number Atom Reactivity Ionic Practical Lesson Element Neutron Precipitate Covalent Electron Isotope Spectra Document1