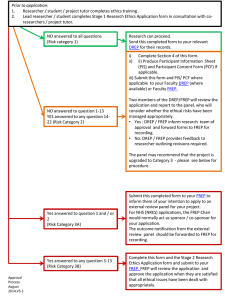
Chapter 5. Coevolution in diploid, sexual systems Biological
advertisement

Chapter 5. Coevolution in diploid, sexual systems
Biological Motivation
So far, when we have studied coevolution in a genetically explicit context, we have invariably
assumed that the interacting species were haploid. Although there are many important species
interactions occurring between haploid species (e.g., XX), interactions between diploid species are
abundant and equally important. For instance, the interactions between Wild Flax and Flax Rust we
studied in Chapters 2 and 4 actually involve species that are diploid despite our earlier genetic
assumptions. How then, might our previous predictions about coevolutionary dynamics and outcomes
change if we integrate the additional complexities of diploidy into our earlier coevolutionary models?
Our central goal in this chapter will be to answer this broad question by evaluating when and where
diploidy matters for the process of coevolution. Specifically, we will develop and analyze diploid models
and use them to revisit the three questions we posed in Chapter 2 when we first explored
coevolutionary interactions mediated by major genes in haploid systems.
Key Questions:
Does coevolution maintain genetic polymorphism?
Can coevolution explain variation in infectivity within populations?
Should coevolution cause infection rates to differ among populations?
Building a diploid model of coevolution
Let’s begin by returning to the interaction between wild Flax and Flax-Rust that we considered
previously. Although we have studied this system in some depth already, we have done so under the
assumption that both Flax and Rust are haploid — an assumption that we know to be false. Our goal
here is to develop a diploid model of this interaction and use it to predict the dynamics and outcomes of
coevolution. Fortunately, even though our species are now diploid, we can follow a sequence of steps
almost identical to those we employed in Chapter 2 to develop our coevolutionary model. The only
novel challenge we face is the need to study how Genotype frequencies change rather than only Allele
frequencies. Although this requires that we specify fitnesses for three genotypes rather than only two
alternative alleles, if we are willing to assume individuals mate at random and have very large
population sizes, this is really the only additional complexity diploidy brings to the table.
Specifically, let’s imagine that prior to natural selection there are NX,RR homozygous flax
individuals carrying two copies of the resistant R gene, NX,Rr heterozygous flax individuals carrying one
copy of the resistant R gene and one copy of the susceptible r gene, and NX,rr homozygous flax
individuals carrying two copies of the susceptible r gene. In addition, let’s assume that the probability a
homozygous resistant flax individual survives to reproduce is WX,RR, whereas for a heterozygous flax
individual the survival probability is WX,Rr, and for a homozygous susceptible flax individual the
probability is WX,rr. Just prior to reproduction, then, the number of homozygous resistant individuals is:
Mathematica Resources: http://www.webpages.uidaho.edu/~snuismer/Nuismer_Lab/the_theory_of_coevolution.htm
′
𝑁𝑋,𝑅𝑅
= 𝑁𝑋,𝑅𝑅 𝑊𝑋,𝑅𝑅 = 𝑋𝑅𝑅 𝑁𝑋 𝑊𝑋,𝑅𝑅
(1a)
the number of heterozygous individuals is:
′
𝑁𝑋,𝑅𝑟
= 𝑁𝑋,𝑅𝑟 𝑊𝑋,𝑅𝑟 = 𝑋𝑅𝑟 𝑁𝑋 𝑊𝑋,𝑅𝑟
(1b)
the number of homozygous susceptible individuals is:
′
𝑁𝑋,𝑟𝑟
= 𝑁𝑋,𝑟𝑟 𝑊𝑋,𝑟𝑟 = 𝑋𝑟𝑟 𝑁𝑋 𝑊𝑋,𝑟𝑟
(1c)
and the total number of individuals is:
̅𝑋
𝑁𝑋′ = 𝑋𝑅𝑅 𝑁𝑋 𝑊𝑋,𝑅𝑅 + 𝑋𝑅𝑟 𝑁𝑋 𝑊𝑋,𝑅𝑟 + 𝑋𝑟𝑟 𝑁𝑋 𝑊𝑋,𝑟𝑟 = 𝑁𝑋 𝑊
(1d)
̅𝑋 is the average probability a flax individual survives to reproduce, or the population mean
where 𝑊
fitness.
Since we now know how many flax individuals of each genotype will survive to reproduce,
calculating genotype frequencies after selection is straightforward:
′
𝑋𝑅𝑅
=
′
𝑋𝑅𝑟
=
′
𝑋𝑟𝑟
=
′
𝑁𝑋,𝑅𝑅
′
𝑁𝑋
′
𝑁𝑋,𝑅𝑟
′
𝑁𝑋
′
𝑁𝑋,𝑟𝑟
′
𝑁𝑋
=
𝑋𝑅𝑅 𝑁𝑋 𝑊𝑋,𝑅𝑅
̅𝑋
𝑁𝑋 𝑊
=
𝑋𝑅𝑟 𝑁𝑋 𝑊𝑋,𝑅𝑟
̅𝑋
𝑁𝑋 𝑊
=
𝑋𝑟𝑟 𝑁𝑋 𝑊𝑋,𝑟𝑟
̅𝑋
𝑁𝑋 𝑊
=
=
=
𝑋𝑅𝑅 𝑊𝑋,𝑅𝑅
̅𝑋
𝑊
(2a)
𝑋𝑅𝑟 𝑊𝑋,𝑅𝑟
̅𝑋
𝑊
(2b)
𝑋𝑟𝑟 𝑊𝑋,𝑟𝑟
̅𝑋
𝑊
(2c)
Next, we need to model what happens to these genotype frequencies when the surviving individuals
mate and reproduce. The good news is that if we are willing to assume individuals of both species mate
at random, undergo sexual reproduction, and that population sizes are very large, our model simplifies
substantially and we need only follow change in a single allele frequency to understand evolution within
the Flax population. The reason for this is that if mating is sexual and occurs at random, the genotype
frequencies X following mating but before selection are given by their Hardy Weinberg proportions such
that equations (2) can be re-written as:
′
𝑋𝑅𝑅
=
2
𝑝𝑋
𝑊𝑋,𝑅𝑅
̅𝑋
𝑊
(3a)
′
𝑋𝑅𝑟
=
2𝑝𝑋 (1−𝑝𝑋 )𝑊𝑋,𝑅𝑟
̅𝑋
𝑊
(3b)
′
𝑋𝑟𝑟
=
(1−𝑝𝑋 )2 𝑊𝑋,𝑟𝑟
̅𝑋
𝑊
(3c)
̅𝑋 = 𝑝𝑋2 𝑊𝑋,𝑅𝑅 + 2𝑝𝑋 (1 − 𝑝𝑋 )𝑊𝑋,𝑅𝑟 + (1 − 𝑝𝑋 )2 𝑊𝑋,𝑟𝑟 . Finally, recognizing that the frequency
where 𝑊
of the R allele can also be written as 𝑝𝑋 = 𝑋𝑅𝑅 + 𝑋𝑅𝑟 ⁄2 allows the frequency of the resistant R allele to
be calculate in the subsequent generation:
2
𝑝𝑋′ =
2
𝑝𝑋
𝑊𝑋,𝑅𝑅 +𝑝𝑋 (1−𝑝𝑋 )𝑊𝑋,𝑅𝑅
̅𝑋
𝑊
(4)
If we now subtract the frequency of the resistant R allele within the previous generation, 𝑝𝑋 , from
Equation (4) we arrive at an expression for evolutionary change over a single generation:
∆𝑝𝑋 = 𝑝𝑋′ − 𝑝𝑋 =
2
𝑝𝑋
𝑊𝑋,𝑅𝑅 +𝑝𝑋 (1−𝑝𝑋 )𝑊𝑋,𝑅𝑟
̅𝑋
𝑊
− 𝑝𝑋 =
̅ 𝑋)
𝑝𝑋 (𝑝𝑋 𝑊𝑋,𝑅𝑅 +(1−𝑝𝑋 )𝑊𝑋,𝑅𝑟 −𝑊
̅
𝑊𝑋
(5)
An identical sequence of mathematical operations (and assumptions) yields a parallel expression for the
frequency of the virulent V gene in the rust:
∆𝑝𝑌 = 𝑝𝑌′ − 𝑝𝑌 =
2
𝑝𝑌
𝑊𝑌,𝑉𝑉 +𝑝𝑌 (1−𝑝𝑌 )𝑊𝑌,𝑉𝑣
̅𝑌
𝑊
− 𝑝𝑌 =
̅ 𝑌)
𝑝𝑌 (𝑝𝑌 𝑊𝑌,𝑉𝑉 +(1−𝑝𝑌 )𝑊𝑌,𝑉𝑣 −𝑊
̅
𝑊𝑌
(6)
We now have general expressions for the change in the frequencies of relevant alleles in flax and rust.
For these expressions to be useful to us, however, we must specify the fitness of the various genotypes.
Our general approach to specifying fitness will be identical to that which we took when we
explored haploid models in Chapter 2. Specifically, we will assume each individual encounters one other
randomly selected individual over its lifetime. For Flax individuals, an encounter with a rust that leads to
a successful infection reduces individual fitness by an amount s, and for a Rust individual, an encounter
with a Flax that fails to produce a successful infection reduces individual fitness by some amount s.
NOTE THAT THIS IS SUBTLY DIFFERENT THAN OUR ASSUMPTIONS OF YORE…
𝑊𝑋,𝑅𝑅 = 1 − 𝑠𝑋 (𝑝𝑌2 𝛼𝑅𝑅,𝑉𝑉 + 2𝑝𝑌 (1 − 𝑝𝑌 )𝛼𝑅𝑅,𝑉𝑣 + (1 − 𝑝𝑌 )2 𝛼𝑅𝑅,𝑣𝑣 )
(7a)
𝑊𝑋,𝑅𝑟 = 1 − 𝑠𝑋 (𝑝𝑌2 𝛼𝑅𝑟,𝑉𝑉 + 2𝑝𝑌 (1 − 𝑝𝑌 )𝛼𝑅𝑟,𝑉𝑣 + (1 − 𝑝𝑌 )2 𝛼𝑅𝑟,𝑣𝑣 )
(7a)
𝑊𝑋,𝑟𝑟 = 1 − 𝑠𝑋 (𝑝𝑌2 𝛼𝑟𝑟,𝑉𝑉 + 2𝑝𝑌 (1 − 𝑝𝑌 )𝛼𝑟𝑟,𝑉𝑣 + (1 − 𝑝𝑌 )2 𝛼𝑟𝑟,𝑣𝑣 )
(7a)
𝑊𝑌,𝑉𝑉 = 1 − 𝑠𝑌 (1 − 𝑝𝑋2 𝛼𝑅𝑅,𝑉𝑉 − 2𝑝𝑋 (1 − 𝑝𝑋 )𝛼𝑅𝑟,𝑉𝑉 − (1 − 𝑝𝑋 )2 𝛼𝑟𝑟,𝑉𝑉 )
(8a)
𝑊𝑌,𝑉𝑣 = 1 − 𝑠𝑌 (1 − 𝑝𝑋2 𝛼𝑅𝑅,𝑉𝑣 − 2𝑝𝑋 (1 − 𝑝𝑋 )𝛼𝑅𝑟,𝑉𝑣 − (1 − 𝑝𝑋 )2 𝛼𝑟𝑟,𝑉𝑣 )
(8a)
𝑊𝑌,𝑣𝑣 = 1 − 𝑠𝑌 (1 − 𝑝𝑋2 𝛼𝑅𝑅,𝑣𝑣 − 2𝑝𝑋 (1 − 𝑝𝑋 )𝛼𝑅𝑟,𝑣𝑣 − (1 − 𝑝𝑋 )2 𝛼𝑟𝑟,𝑣𝑣 )
(8a)
Developing mathematical expressions for coevolutionary change requires only that we
substitute fitness functions (7-8) into evolutionary recursions (5-6) and replace the general interaction
matrix entries 𝛼𝑖,𝑗 with values appropriate for the interaction between Flax and Flax-Rust. But what are
the appropriate entries now that we are dealing with a diploid system and must specify values of 𝛼 for
the 9 different combinations of Flax and Rust genotypes that can possibly encounter one another?
MORE TEXT ON DOMINANCE!
3
1 1
𝛼 = [0 0
0 0
1
1]
1
(9)
where columns are defined by the Flax genotypes {RR, Rr, rr} and rows are defined by Rust genotypes
{VV, Vv, vv}. DOMINANCE
Making these substitutions and simplifying yields the following expressions for the change in
frequency of flax R and rust Vir alleles:
∆𝑝𝑋 =
2
2
𝑠𝑋 𝑝𝑋 𝑞𝑋
(2𝑝𝑌 𝑞𝑌 +𝑞𝑌
)
̅𝑋
𝑊
(10a)
∆𝑝𝑌 =
2
2
𝑠𝑌 𝑝𝑌
𝑞𝑌 (𝑝𝑋
+2𝑝𝑋 𝑞𝑋 )
̅𝑌
𝑊
(10b)
where 𝑞𝑋 = (1 − 𝑝𝑋 ) and 𝑞𝑌 = (1 − 𝑝𝑌 ) are the frequencies of flax r and pathogen v alleles,
respectively. What can we learn from these relatively simple expressions?
Analyzing the model
One potentially important difference is that, now, the rate of coevolution in the Flax depends on
a term 𝑝𝑋 𝑞𝑋2 rather than 𝑝𝑋 𝑞𝑋 as in the haploid case and the rate of coevolution in the Rust depends on
a term 𝑝𝑌2 𝑞𝑌 rather than 𝑝𝑌 𝑞𝑌 as we saw in the haploid case. The reason for this difference is that the
rate of evolution in diploid systems depends on dominance. For the Flax population, resistance to the
Rust is dominant (see matrix alpha), and is expressed in both heterozygotes and homozygotes. As a
consequence, selection can “see” even very rare resistant alleles in the Flax population and effectively
increase them in frequency. In contrast, the ability to overcome Flax resistance within the Rust
population is conferred by a virulence allele which is recessive (see matrix alpha), and expressed only in
homozygous individuals. Thus, only rust individuals homozygous for this virulent allele can overcome
Flax resistance and these homozygous genotypes will be very, very rare when the virulent allele first
arises within the population (hence the 𝑝𝑌2 term). Putting all this together suggests that when both
resistant and virulent alleles are initially rare, the Flax population should be able to evolve resistance
more rapidly than the Rust population can evolve to overcome it (Figure 1).
Answers to key questions
Genetic variation is never maintained
Ultimately variation in infectivity will be lost
infectivity should, ultimately be constant across populations
SO… QUALITATIVELY, NOTHING DIFFERS FROM THE HAPLOID CASE… What about quantitatively?
New Questions Arising:
4
One potentially important difference is that, now, the Flax population should be able to evolve
resistance more rapidly than the Rust population can evolve to overcome it (Figure 1). Although
equations (5) show that this advantage experience by the Flax population is transient, it could have
important consequences if we were to simultaneously consider demography (REFS?), or if we allowed
for the repeated origin of novel resistance and virulence alleles (REFS). SUGGESTS DOMINANCE AND
PLOIDY COULD HAVE INTERESTING CONSEQUENCES FOR COEVOLUTION raising several important
questions:
What other patterns of dominance are possible in coevolving systems?
How do these alternative patterns of dominance influence coevolution?
How do diploidy and mode of reproduction interact??? ie. CLONALITY AND DIPLOIDY OR…
MIXED PLOIDY SYSTEMS… FOR INSTANCE DAPHNIA AND PASTUERIA
In the next two sections, we will generalize our simple model in ways that allow us to answer these
questions.
Generalizations
Generalization 1: What other patterns of dominance are possible in coevolving systems?
Although the pattern of dominance considered so far for the gene-for-gene interaction between
Flax and Flax-rust is commonly observed between plants and fungal pathogens (REFS), there are many
other important interactions for which dominance is unknown. In these cases, it is worth considering
alternative forms of dominance that might arise from what we know about the molecular mechanisms
underpinning the interaction. For instance, the medically important interaction between the snail,
Biomphalaria glabrata and its schistosome parasite, Schistosoma mansoni, exhibits significant variability
in outcome depending on the genotypes of the interacting individuals (REFS). Thus, the potential clearly
exists for coevolution to drive changes in genotype frequencies within the interaction. However, unlike
the interaction between Flax and Flax-Rust we considered previously, the pattern of dominance within
this interaction is unclear. Instead, making progress modeling this interaction requires using what we
know about the molecular mechanisms of infection and resistance to infer the structure of the alpha
matrix from this information.
Recent infection experiments conducted between these species have identified the interaction
between S. mansoni mucin molecules and B. glabrata FREP molecules as a candidate mechanism
mediating the genetic specificity of the interaction (Mitta et al. 2012). Specifically, for the host, B.
glabrata to potentially clear an infection by encapsulating the parasite, its FREP molecules must be able
to successfully bind to mucin molecules produced by the parasite. Thus, it appears that this interaction
is, at least partially, mediated by a mechanism of targeted recognition where the host must match the
parasite if infection is to be prevented (Dybdahl et al. 2014). This sort of mechanism has generally been
modeled as an inverse matching alleles model, and as long as both species are haploid, it is
straightforward to write down an appropriate interaction matrix, α, as we did in Chapter 2 (DID WE DO
THAT?). If the interacting species are diploid (as is the case for the interaction between S. mansoni and
5
B. glabrata), however, a bit more thought must be given to the pattern of dominance and the structure
of the interaction matrix, α, that results.
If we are willing to ignore some of the true genetic complexities underlying the production of
FREP and Mucin molecules and assume both proteins are encoded by a single genetic locus with two
possible alleles (A and a in B. glabrata and B and b in S. mansoni) we can begin to predict the phenotype
of individual snails and schistosomes and the outcome of interactions. If both FREPS and Mucins are
monomeric proteins, phenotypes of individual snails and schistosomes might be expected to be as
depicted in Table 1.
Table 1. Mapping between genotype and phenotype (monomeric proteins)
B. glabrata
FREP
S. mansoni
Mucin
genotype
phenotype
genotype
phenotype
AA
A
BB
B
1/2 B and ½ b
Aa
1/2 A and ½ a
Bb
aa
a
bb
b
If we now assume that once the schistosome enters its snail host it produces a single Mucin molecule
which is confronted with a single snail FREP molecule, successful infection results only when this FREP
and Mucin do not match. Otherwise, the snail recognizes and clears the infection. In such a scenario, the
interaction matrix would take the following form:
0
1/2
1
𝛼 = [1/2 1/2 1/2]
1
1/2
0
(11)
where columns are defined by B. glabrata genotypes {AA, Aa, aa}, rows are defined by S. mansoni
genotypes {BB, Bb, bb}, and the entries indicate the probability of successful infection. What if, however,
upon entering a snail each schistosome produces many Mucin molecules and the snail produces many
FREP molecules? Perhaps in such a case, a host immune response is triggered and the infection cleared
if only a single FREP matches a single Mucin such that infection occurs only when no FREP and Mucin
molecules match one another. As the number of random encounters between FREP and Mucin
molecules becomes very large, the interaction matrix approaches:
0 0
𝛼 = [0 0
1 0
1
0]
0
(12)
Obviously, the infection matrices (11-12) are extreme cases and reality may lie somewhere in between.
What is really important, however, is that anywhere between these two extreme cases an interesting
and important pattern of dominance emerges for this interaction. Specifically, what we see is that as
long as encounters among snail and schistosome genotypes occur at random and all genotypes are
present, heterozygous snails are more resistant, on average, than homozygous snails. This represents a
6
case of effective overdominance. Within the schistosome population, however, heterozygous individuals
are less infective, on average, than homozygous schistosomes. This represents a case of effective
underdominance.
If, in contrast, both FREPS and Mucins are dimeric proteins, phenotypes of individual snails and
schistosomes might look something more like what is shown in Table 2.
Table 2. Mapping between genotype and phenotype (dimeric proteins)
B. glabrata
FREP
S. mansoni
Mucin
genotype
phenotype
genotype
phenotype
AA
AA
BB
BB
Aa
1/4 AA: 1/2 Aa: 1/4 aa
Bb
1/4 BB: ½ Bb: 1/4 bb
aa
aa
bb
bb
As before, we first consider what happens if snail and schistosome each produce only a single FREP and
Mucin molecule. In such a case, infection results only when this pair of FREP and Mucin molecules does
not match, leading to the following interaction matrix:
0
3/4
1
𝛼 = [3/4 5/8 3/4]
1
3/4
0
(12)
where columns are defined by B. glabrata genotypes {AA, Aa, aa} and rows are defined by S. mansoni
genotypes {BB, Bb, bb}. Next, we consider what happens if both snail and schistosome produce a very
large number of FREP and Mucin molecules. In such a case, infection results only when no matches
occur between any of these FREP and Mucin molecules. In the limit of a very large number of random
encounters between Freps and Mucins the interaction matrix then approaches:
0 0
𝛼 = [0 0
1 0
1
0]
0
(13)
In contrast to what we observed for monomeric proteins, we now see that the effective pattern of
dominance that emerges depends on the details of immune defense. Specifically, if snail immune
defense relies on a single encounter between FREP and Mucin molecules, interaction matrix (12) shows
that heterozygous snails are more resistant than homozygous snails and heterozygous schistosomes are
more infective than homozygous schistosomes. In such a case, then, we would observe overdominance
for both infectivity and resistance. If, instead, a very large number of Mucin and FREP molecules
encounter one another at random, interaction matrix (13) shows that heterozygous snails are more
resistant than homozygous snails but that heterozygous schistosomes are less infective than
homozygous schistosomes.
7
Comparing the interaction matrices we have derived for various possible scenarios shows that
the patterns of dominance we expect for infectivity and resistance depend quite a bit on molecular
details of immune molecule structure and the details of immune interactions (Table 3).
Table 3. Expected patterns of effective dominance
Mucin and FREP
Number of FREP and Mucin
structure
molecules produced
Monomeric
One
Monomeric
Many
Dimeric
One
Dimeric
Many
Schistosome
Infectivity
No dominance
Strongly
Underdominant
Weakly
Overdominant
Strongly
Underdominant
Snail Resistance
No Dominance
Strongly
Overdominant
Weakly
Overdominant
Strongly
Overdominant
Clearly, these results show that the simple pattern of dominance we explored for the interaction
between wild Flax and Flax-Rust is not likely to be general across other types of coevolving systems. In
the next section, we explore how the different possible patterns of dominance shown in Table 3
influence the dynamics and outcome of coevolution.
Generalization 2: How do these alternative patterns of dominance influence coevolution?
Interaction matrix 1. HOST HAS A and a, Parasite has B and b
Conclusions and Synthesis
ONE OF THE GREATEST STANDING CHALLENGES IN COEVOLUTIONARY GENETICS IS UNDERSTANDING
PATTERNS OF DOMINANCE.
XXXXXXXXXXXXXs such that individuals with genotype AA make only FREP molecules with phenotype
“A”, heterozygous Aa individuals make FREP molecules with phenotype “A” and also FREP molecules
with phenotype “A”, and individuals with genotype aa make phenotype “a”. If
The interaction between Biomphalaria glabrata and Schistosoma mansoni, (Mitta et al. 2012)
FREPS and Mucins. The host FREP must match the parasite mucin in order for the host to recognize and
potentially clear the infection. What pattern of dominance might arise from a molecular mechanism like
this? Unfortunately, without knowing how these proteins are constructed Assuming the FREP and Mucin
8
are the product of a single locus, it is logical manifestWe might imagine, then, that if both Mucins and
FREPS are monomeric proteins, the outcome would be XX and XX. However, if they were dimeric, more
outcomes become possible…
1 1
𝛼 = [0 0
0 0
1
1]
1
FREPS AND MUCINS!
HERE WE EXPLORE SYSTEM X DOMINANCE
“Unlike other trematodes, the schistosomes are dioecious, i.e., the sexes are separate. The two
sexes display a strong degree of sexual dimorphism, and the male is considerably larger than the
female. The male surrounds the female and encloses her within his gynacophoric canal for the entire
adult lives of the worms, where they reproduce sexually.”
Biomphalaria glabrata are simultaneous hermaphrodites but apparently diploid…
Generalization 2: How do these alternative patterns of dominance influence coevolution?
HERE WE EXPLORE COEVOLUTION BETWEEN S
Generalization 3: MANY SYSTEMS HAVE MIXED PLOIDY… HOW CAN WE HANDLE THAT? How do
diploidy and mode of reproduction interact??? ie. CLONALITY AND DIPLOIDY OR… MIXED PLOIDY
SYSTEMS… FOR INSTANCE DAPHNIA AND PASTUERIA
Conclusions and Synthesis
9
10
References
Figure Legends
Dybdahl, M. F., C. E. Jenkins, and S. L. Nuismer. 2014. Identifying the Molecular Basis of Host-Parasite
Coevolution: Merging Models and Mechanisms. AMERICAN NATURALIST 184:1-13.
Mitta, G., C. M. Adema, B. Gourbal, E. S. Loker, and A. Theron. 2012. Compatibility polymorphism in
snail/schistosome interactions: From field to theory to molecular mechanisms. Developmental
and Comparative Immunology 37:1-8.
11