Towards the rational design of freestanding photoelectrochemical
advertisement
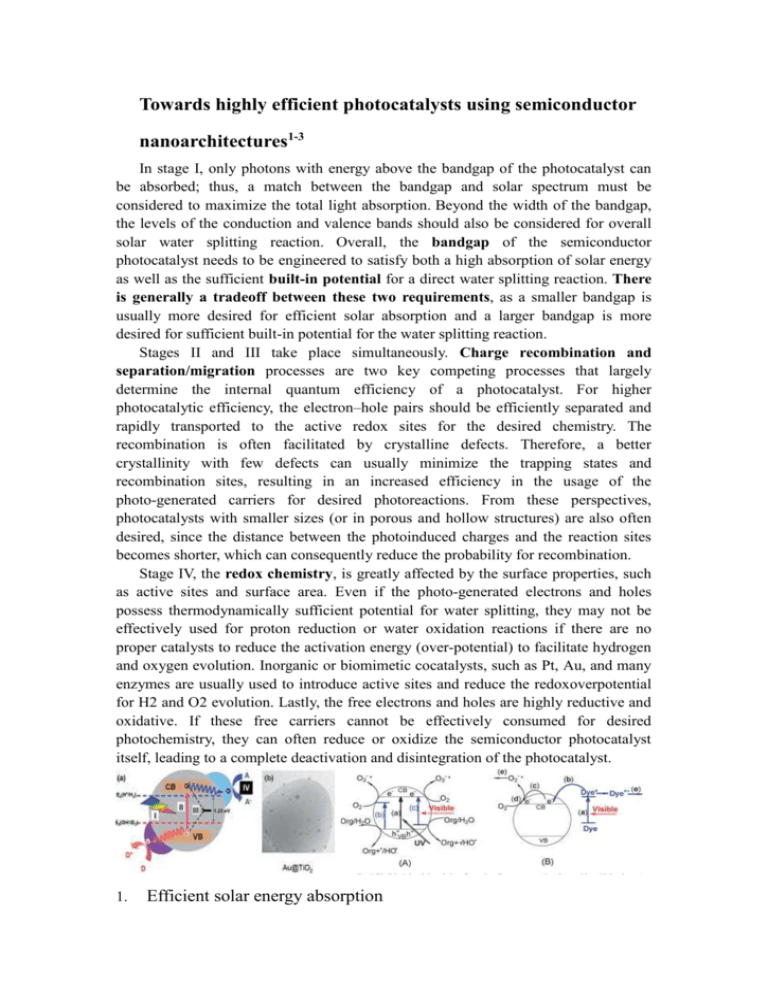
Towards highly efficient photocatalysts using semiconductor nanoarchitectures1-3 In stage I, only photons with energy above the bandgap of the photocatalyst can be absorbed; thus, a match between the bandgap and solar spectrum must be considered to maximize the total light absorption. Beyond the width of the bandgap, the levels of the conduction and valence bands should also be considered for overall solar water splitting reaction. Overall, the bandgap of the semiconductor photocatalyst needs to be engineered to satisfy both a high absorption of solar energy as well as the sufficient built-in potential for a direct water splitting reaction. There is generally a tradeoff between these two requirements, as a smaller bandgap is usually more desired for efficient solar absorption and a larger bandgap is more desired for sufficient built-in potential for the water splitting reaction. Stages II and III take place simultaneously. Charge recombination and separation/migration processes are two key competing processes that largely determine the internal quantum efficiency of a photocatalyst. For higher photocatalytic efficiency, the electron–hole pairs should be efficiently separated and rapidly transported to the active redox sites for the desired chemistry. The recombination is often facilitated by crystalline defects. Therefore, a better crystallinity with few defects can usually minimize the trapping states and recombination sites, resulting in an increased efficiency in the usage of the photo-generated carriers for desired photoreactions. From these perspectives, photocatalysts with smaller sizes (or in porous and hollow structures) are also often desired, since the distance between the photoinduced charges and the reaction sites becomes shorter, which can consequently reduce the probability for recombination. Stage IV, the redox chemistry, is greatly affected by the surface properties, such as active sites and surface area. Even if the photo-generated electrons and holes possess thermodynamically sufficient potential for water splitting, they may not be effectively used for proton reduction or water oxidation reactions if there are no proper catalysts to reduce the activation energy (over-potential) to facilitate hydrogen and oxygen evolution. Inorganic or biomimetic cocatalysts, such as Pt, Au, and many enzymes are usually used to introduce active sites and reduce the redoxoverpotential for H2 and O2 evolution. Lastly, the free electrons and holes are highly reductive and oxidative. If these free carriers cannot be effectively consumed for desired photochemistry, they can often reduce or oxidize the semiconductor photocatalyst itself, leading to a complete deactivation and disintegration of the photocatalyst. 1. Efficient solar energy absorption (the bandgap engineering of the photocatalysts to satisfy the requirements for both efficient solar energy absorption and sufficient built-in potential for redox reactions) Although TiO2 based photocatalysts can function as effective photocatalysts, they cannot be used for effective solar energy harvesting and conversions. Owing to its large bandgap ( Eg>3eV), TiO2 can only absorb photons with light wavelengths shorter than about 400 nm in the UV or near-UV wavelength regime, which accounts for less than 5% of the total solar energy irradiation through the atmosphere to the sea level. Significant challenges remain in developing a visible light driven photocatalyst with high efficiency and chemical stability. To this end, considerable effort has been placed on improving the solar absorption of the photocatalysts. Overall, three typical approaches have been explored for the enhanced light absorption in the visible light region, which include: (1) chemical doping of TiO2, such as incorporation of additional metal or nonmetal species ( e.g. , nickel, vanadium, chromium, platinum, nitrogen, tungsten, fluorine, sulfur, etc. ) (2) the exploration of alternative narrow bandgap semiconductor photocatalysts, such as Si, InP, CdSe, and GaZnON; and (3) the loading of the visible light sensitizer, such as organic dye, metal nanoparticles, or a narrow bandgap semiconductor. Recently, Chen et al. developed an interesting approach to significantly enhance the visible light absorption (up to 1200 nm) by intentionally creating a disordered shell of several atomic layers of nanophase TiO2 using a hydrogenation method to create a so-called ‘‘black TiO2’’. The disorder-engi-neered black TiO2 exhibited a higher efficiency of the visible-light-driven photocatalysis compared to traditional white TiO2. Soon after, Y. Li and co-workers obtained a solar-to-hydrogen efficiency of 1.63% by using hydrogen treated TiO2 nanowires for photoelectrochemical water splitting, which is the best value for a TiO2 photoanode. A great number of new photocatalytic materials have been proposed as potential substitutes of TiO2; many of them follow two possible ways. One is searching for native semiconductors with a bandgap covering the visible light region; a -Fe2O3 (hematite), CdS, BiVO4, and Ag3PO4 are well known native visible-light-driven photocatalysts. The other is the solid solutions of photocatalysts with the same crystal structure. Since2005, Domen’s group has systematically studied the GaN– ZnO solid solutions for photocatalytic purposes, which have been demonstrated split water into hydrogen and oxygen under visible light irradiation. Narrower bandgap semiconductors, dye molecules, and/or metal nanoparticles are widely used as the sensitizers on the surface of photocatalysts to capture additional visible or infrared light and consequently enhance the photocatalytic efficiency. Only the narrow bandgap sensitizer can be excited to generate electrons in their conduction band under the visible light irradiation. For an efficient electron transfer between the sensitizer and photocatalyst, the energy level of the conduction band of the photocatalyst must be lower than that of the sensitizers. Thus, the electrons created in sensitizers are subsequently injected into the photocatalyst conduction band to perform a reduction reaction. If the energy level of the valence band of the sensitizer is higher than that of the photocatalyst, the photoinduced holes are localized inside the sensitizer. In this heterostructure, several advantages can be obtained: (1) an enhanced visible light absorption; (2) a more effective charge separation; (3) a rapid charge transfer to catalyst; and (4) a longer lifetime of the charge carriers. Several semiconductor sensitizers with a narrow bandgap were shown in which have been proven to enhance the solar energy harvesting efficiency in the visible light region. Organic dye molecules have also been employed as sensitizers for semiconductor photocatalysts due to their active light absorption in the visible wavelength regime. The dye-sensi-tizers used for solar cell and photocatalytic reactions have attracted more and more attention because of their stability, low cost, and high efficiency. Lastly, some metal nanoparticles, with surface plasmon induced absorption in the visible light region, could also be loaded on the surface of wide bandgap photocatalysts ( e.g. ,TiO2) to enhance their light harvesting efficiency. The surface plasmon resonance is created by the coherent oscillation of the metal conduction band electrons upon excitation by irradiating light. A dipolar oscillation of all the electrons induces strong absorption of the electromagnetic energy when its frequency becomes in resonance with the electron oscillation. The frequency of the surface plasmon absorption highly depends on the size and morphology of the metal nanoparticles, as well as their dielectric environment. For most metals such as In, Sn, Pb, and Cd, the plasmon frequency lies in the UV region with a broad and poor absorption band, while noble metals such as Cu, Ag, and Au exhibit a strong visible-light plasmon resonance. The tunable plasmonic absorption bands of various Au and Ag nanostructures throughout the visible range make them ideal candidates for enhancing photocatalytic absorption and efficiency. 2. Separation and transportation of the photogenerated charge carriers (heterostructured junctions with internal built-in electrical potential to effectively direct electron–hole separation and transportation) The formation of semiconductor heterostructure (formed by the direct contact of semiconductors A and B) is an effective approach to promote charge separation for improved photo-catalytic activity. Considering a type-II band alignment, the energy gradient existing at the interfaces tends to spatially separate electrons and holes on different sides of the hetero-junction, where electrons may be confined to one side and holes to the other. The spatially localized charges in the type-II nanostructures should make these materials more suitable for photocatalytic applications. The semiconductor p–n junction is another effective architec-ture for the highly efficient charge separation and transportation. In general, when the p- and n-type semiconductor materials are in contact, they form a p–n junction with a space-charge region at the interfaces due to the diffusion of electrons and holes, and thus create a built-in electrical potential that can direct the electrons and holes to travel in the opposite direction (Fig. 11). When the p–n heterojunction is irradiated with photon energy higher or equal to the bandgaps of the photocatalysts, the photogenerated electron–hole pairs can be quickly separated by the built-in electric field within the space charge region. Driven by the electric field, the electrons are transferred to the conduction band of the n-type semiconductors and the holes to the valence band of the p-type semiconductors. Metal–semiconductor contacts with a potential offset have also been explored to facilitate the separation and transportation of the photogenerated electron–hole pairs. In a way much similar to the semiconductor junctions, the potential offset at a metal– semiconductor contact can create a space charge region and a built-in potential to drive the separation and transportation of photogenerated electron–hole pairs. The eventual impact of metal–semiconductor contacts is however much more complicated due to other possible contributions of metal clusters such as co-catalyst effect, plasmonic resonance effect and charge trapping effect. 3. Redox cocatalysts for efficient charge utilization (the integration with a proper cocatalyst to reduce the redox overpotential) The redox cocatalyst is an important factor determining the eventual efficiency of the photocatalytic process because it dictates the efficiency in the utilization of photogenerated charges for actual redox reactions. Due to the limitation of the activation energy (overpotential), the photo-generated and separated electrons and holes may not be effectively utilized for the desired reduction or oxidation reaction without proper cocatalysts to decrease the overpotential. Inorganic or biomimetic cocatalysts, such as Pt, Au, and many enzymes are widely used to reduce the redox overpotential. In general, the cocatalysts can be broadly classified into two groups: reduction catalysts and oxidation catalysts, and more specifically for the water splitting reaction: hydrogen evolution catalysts and oxygen evolution catalysts. Although it is obvious that the effective integration of theredox cocatalysts can play a rather important role in the efficiency and stability of photocatalytic systems, little systematic effort has been made for this problem to date, largely due to the difficulties in selectively integrating the reduction and oxidation catalysts with the designed architecture to facilitate the desired charge transfer process. An enhanced photocatalytic activity is expected by loading both reduction and oxidation cocatalysts, compared to photocatalysts modified with only a single cocatalyst. Aided by the cocatalysts, the charge carriers are eventually consumed on the interfaces of cocatalysts/solution. The spatially separated redox active sites can effectively avoid the back reaction, which is an essential issue for photocatalytic water splitting. 4. Reliability of the photocatalysts (the electrochemical stability of the photocatalyst.) Chemical stability is the most critical issue of semiconductor photocatalysts dispersed in an aqueous solution under light irradiation. Photocorrosion, degradation of the photocatalyst under light irradiation, is considered the main reason for the instability of photocatalysts. Some of the oxide and most of the typical semiconductors (e.g. ZnO, ZnS, CdS,etc. ) are susceptible to corrosion under these conditions and therefore can hardly be explored as candidates for effective photocatalysts. At a critical pH, thehydronium ion content will be enough to protonate the catalyst itself, resulting in corrosion. For instance, ZnO under UV light irradiation is known to dissolve readily. This susceptibility significantly decreased the photocatalytic activity of ZnO in aqueous solutions and prevented the further application of ZnO as an effective photocatalyst. Tremendous efforts have been made to improve the photo-stability using various methods. For example, the stability of some photocatalysts was found to increase in the presence of certain inorganic sacrificial reagents, such as the S2-/SO32- system, to avoid the etching process on the photocatalysts themselves. In addition, core–shell structures with an inert oxide or polymer shell are developed to suppress the photocorrosion . 5. Towards the rational design of freestanding photoelectrochemical nanodevices as highly efficient photocatalysts Considering the multiple-step nature of the photocatalytic process, it is important to consider the entire process in order to develop a highly efficient and stable photocatalyst. To this end, we have recently reported a rational strategy to design a new generation of freestanding photoelectrochemical nanodevices as highly efficient and stable photocatalysts. With the seamless integration of multiple functional components in a single nanostructure and precise control of the materials interfaces, this design of photocatalyst offers several key advantages over conventional ones: (1) the internal built-in potential can facilitate efficient electron–hole separation and transportation; (2) the one-dimensional morphology and the insulating shell ensure that the electrons and holes are only directed towards the redox nanocatalysts for the desired redox reactions, and therefore minimize non-productive charge consumption by recombination or undesired side reactions on semiconductor surface; (3) multiple photodiodes can be integrated in series to step-up the electron energy and therefore simultaneously ensure sufficient electrochemical potential for direct water splitting and good absorption overlap with the solar spectrum (Fig. 14b), much like the natural photosynthesis that always uses two photons to drive each electron reaction; and (4)the encapsulation of the semiconductor portion in a protective insulating shell prevents direct electrochemical reactions on the semiconductor surface to ensure the electrochemical stability, and therefore allows flexible selection of semiconductor materials that better match the solar spectrum for efficient solar energy harvesting and conversion. Together, these initial studies for the first time demonstrated the integration of multiple distinct functional components into a single nanostructure to form a standalone photoelectrochemical nanodevice, to enable a photocatalyst that is both efficient and stable throughout theentire solar spectrum. It can thus open a rational avenue to the design and synthesis of a new generation of photoelectrochemical nanosystems with unprecedented efficiency and stability, and will have a broad impact in areas including environmental remediation, artificial photosynthesis and solar fuel production. However, it should be noted that the built in potential (0.3 eV) of this initial system is not enough for direct water splitting or CO2 reduction ( >1.23–1.34 eV); alternative larger bandgap materials or multijunction must be explored to address this critical challenge. Additionally, it would also be important to explore various redox catalysts to facilitate the electron transfer process, reduce overpotential and increase the efficiency of the photocatalytic redox reactions. 6. Conclusion In general, photocatalysis involves a complicated sequence of multiple synergistic or competing steps, including light harvesting, charge generation, separation, transportation, recombination and utilization. Although significant effort has been devoted to optimize each one of these processes, which has led to the development of a wide range of materials and architectures for effective photocatalytic systems, few studies have treated these problems coherently. Very often, the optimization of one parameter could lead to the degradation of the other. It remains a significant challenge to distinguish which factor(s) dominates the overall photocatalytic activity, and it is important to address these problems together. New insights are required to design the light absorption, charge separation and transfer processes and the integration with redox cocatalysts. Rational design of the photocatalyst with the consideration of the whole process could facilitate the creation of highly effective photocatalysts. Semiconductor nanoarchitectures that integrate multiple functional components in the nanoscale could allow efficient charge generation, separation, transportation and utilization to enable a new generation of highly efficient photocatalysts. In a specific example, we have discussed a freestanding photoelectrochemical nanodevice that consisted of a p–n junction nanowire with a protecting shell and two exposed redox cocatalysts, which represents an interesting design for a highly efficient and stable photocatalyst. 附录:通俗来说,欧姆接触和肖特基接触都属于金属和半导体接触的情况, 理想的欧姆接触就像是导线连通,无论正反向测试,电阻都是零,而肖特基接触 在接正向电压时,电阻为零,接反向电压时,电阻无穷大,即:具有单向导电性。 1. Zhou, H. L.; Qu, Y. Q.; Zeid, T.; Duan, X. F., Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy & Environmental Science 2012, 5, 6732-6743. 2. Qu, Y.; Xue, T.; Zhong, X.; Lin, Y.-C.; Liao, L.; Choi, J.; Duan, X., Heterointegration of Pt/Si/Ag Nanowire Photodiodes and Their Photocatalytic Properties. Adv Funct Mater 2010, 20, 3005-3011. 3. Qu, Y.; Liao, L.; Cheng, R.; Wang, Y.; Lin, Y.-C.; Huang, Y.; Duan, X., Rational Design and Synthesis of Freestanding Photoelectric Nanodevices as Highly Efficient Photocatalysts. Nano letters 2010, 10, 1941-1949.






