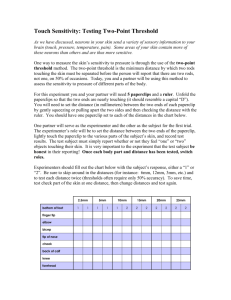
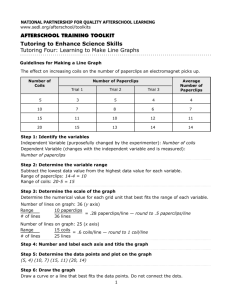
Lab Report Sample ()
advertisement
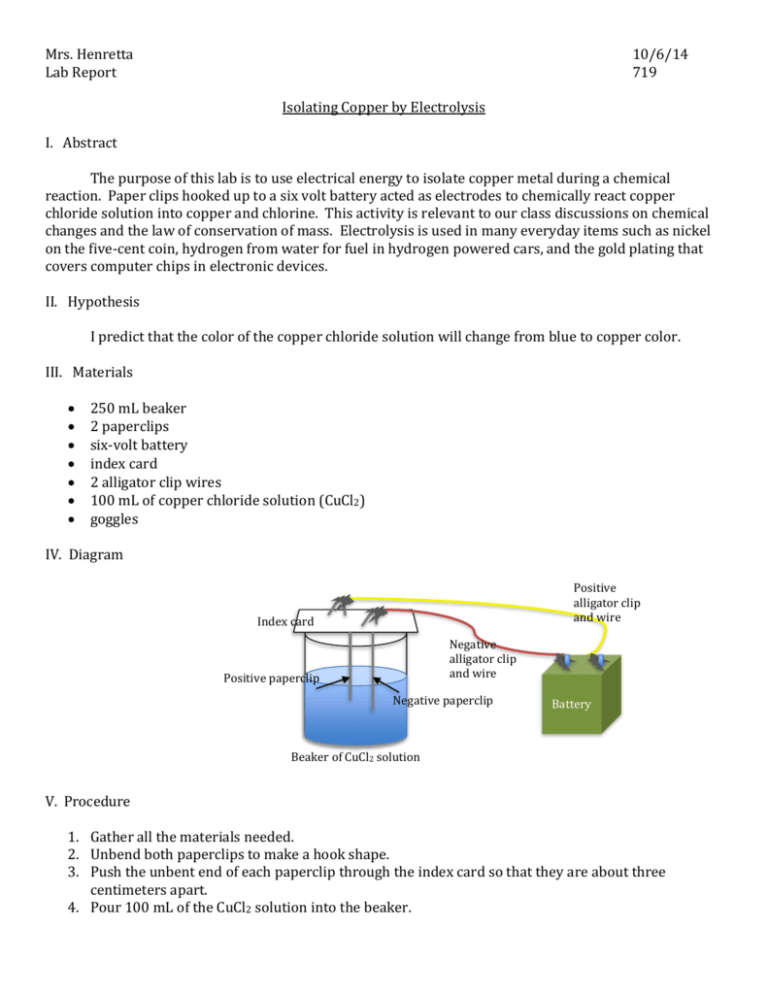
Mrs. Henretta Lab Report 10/6/14 719 Isolating Copper by Electrolysis I. Abstract The purpose of this lab is to use electrical energy to isolate copper metal during a chemical reaction. Paper clips hooked up to a six volt battery acted as electrodes to chemically react copper chloride solution into copper and chlorine. This activity is relevant to our class discussions on chemical changes and the law of conservation of mass. Electrolysis is used in many everyday items such as nickel on the five-cent coin, hydrogen from water for fuel in hydrogen powered cars, and the gold plating that covers computer chips in electronic devices. II. Hypothesis I predict that the color of the copper chloride solution will change from blue to copper color. III. Materials 250 mL beaker 2 paperclips six-volt battery index card 2 alligator clip wires 100 mL of copper chloride solution (CuCl2) goggles IV. Diagram Positive alligator clip and wire Index card Negative alligator clip and wire Positive paperclip Negative paperclip + + Battery Beaker of CuCl2 solution V. Procedure 1. Gather all the materials needed. 2. Unbend both paperclips to make a hook shape. 3. Push the unbent end of each paperclip through the index card so that they are about three centimeters apart. 4. Pour 100 mL of the CuCl2 solution into the beaker. 5. Rest the index card on top of the beaker so that the paperclips about halfway submerged in the solution. 6. Attach one end of an alligator clip wire to one pole of the battery and the other end to one of the paperclips. 7. Repeat step 6 with the other alligator clip wire onto the other battery pole and paperclip. Do not let the paperclips touch each other. 8. Observe for 2-3 minutes. Record any observations. 9. Disconnect the paperclips from the wires and remove the index card and paperclips from the top of the beaker. 10. Observe any changes that may have occurred to the paperclips. 11. Waft the air from the beaker and record any smells. 12. Carefully clean up all materials. VI. Data and Measurements Table 1: Before and After Observations CuCl2 solution Before Blue color, 100 mL After More Greenish Positive Paperclip Silver Small bubbles attached, slightly pointed end Negative paperclip Silver Copper colored, powdery substance; pointed end VII. Observations Before the experiment, the paperclips were silver colored and had a cylindrical shape. Small bubbles began to appear on the positive paperclip during the experiment. A copper colored powder began to form on the negative paperclip. After the experiment, both paperclips were pointed but the negative paperclip was pointed more. The copper chloride solution changed from a blue color to a more greenish-blue color afterwards. When the experiment was finished, a slight odor of chlorine, like in a pool, was detected. VIII. Conclusion 1. Based on my observations, I believe that solid copper was isolated on the negative paperclip and gaseous chlorine was isolated on the positive paperclip. 2. Before the electrolysis, the copper and chlorine were chemically combined into CuCl2. This substance was a bluish liquid. After the chemical reaction, the copper was a brownish solid and the chlorine was a clear gas. 3. I believe that a chemical reaction did take place because the new substances with new properties were formed. 4. This reaction does comply with the law of conservation of mass because no new atoms were created and none were destroyed. The copper and chlorine atoms that used to be combined into a CuCl2 molecule were separated into Cu and Cl. 5. I predict that if the positive and negative alligator clip wires were switched, then solid copper and chlorine gas would form on the opposite paperclips. 6. If I had to do this experiment again, I would use nails instead of paperclips because they are thicker and are made of a different metal. I would also try a more concentrated CuCl2 solution to see if more copper and chlorine gas would form.