a clinical approach to diabetic peripheral neuropathy
advertisement

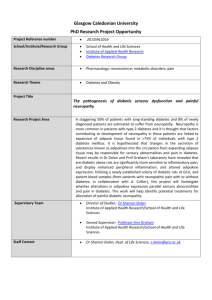
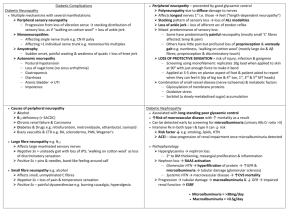
ORIGINAL ARTICLE A CLINICAL APPROACH TO DIABETIC PERIPHERAL NEUROPATHY Riyaz Mohammed1 HOW TO CITE THIS ARTICLE: Riyaz Mohammed. ”A Clinical Approach to Diabetic Peripheral Neuropathy”. Journal of Evidence based Medicine and Healthcare; Volume 1, Issue 16, December 22, 2014; Page: 2009-2016. ABSTRACT: Diabetes mellitus is characterized by increased blood sugars, which can lead to multiple macrovascular and microvascular problem. It is also a known cause of debilitating problem like peripheral neuropathy (PN). This review is done to explain the importance of detecting peripheral neuropathy in a clinical practice. PN causes discomfort and pain in the lower extremities, loss or absence of sensations in the lower extremities leading to postural problems, risk of ulcerations of foot, and reduced quality of life in patients with Type 2 Diabetes. A variety of methods are used to evaluate peripheral nerve functions (objective and subjective measures). It is important to understand how to evaluating PN in routine practice and preventing foot ulcers or fall-related morbidity and mortality in adults with Type 2 Diabetes. DEFINITION: Diabetes mellitus is due to absolute or relative deficiency of Insulin and it is characterized by hyperglycemia. Long standing metabolic derangement is associated with structural and functional changes in multiple organs, particularly in vascular system, which can lead to clinical 'complications' of diabetes. These can affect nervous system, kidneys and eye, kidneys.[1] Prevalence of Type 2 diabetes mellitus: Studies estimated that nearly 250 million people will be affected with diabetes by the year 2020; vastly will have increased Type 2 diabetes mellitus prevalence.[2] It is found that people’ having impaired glucose tolerance are at risk of developing diabetes mellitus i.e. 10 times higher than normoglycemia patients. Multiple studies conducted throughout the world, had found an increase in the incidence of Type 2 diabetes and have concluded that there is an increase in the prevalence, mortality and morbidity secondary to diabetes mellitus especially in the elderly population.[3, 4] World Health Organization (WHO) has conducted a survey in 1995, it was estimated that they were nearly 135 million diabetics, and it was estimated that this figure would double by 2025. Shaw et al., found that the prevalence was high in regions of North America (10.2%) followed by south Asia (6.7%). [5] In India various studies (Misra A et.al 2001, Ramachandran A et al. 2001) were done which concluded that there is increase prevalence of diabetes and its complications.[6,7] It is known that Asian Indians develop type 1 or type 2 diabetes mellitus a decade earlier than the Europeans. Narayan et al had given emphasis on early detection of prediabetes and undetected diabetes and taking effective measures on appropriate treatment.[8] Prevalence of diabetic peripheral neuropathy (DPN): Neuropathic pain is defined as "pain initiated or caused by a primary lesion or dysfunction in the nervous system".[9] In type 2 diabetes J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2009 ORIGINAL ARTICLE mellitus patient the most frequently complication is distal symmetrical polyneuropathy (DSP) or DPN. Tesfaye S, Vileikyte L et .al 2011 suggested that neuropathic pain syndromes have affected nearly 70% of the American population with diabetes.[10] Abbott CA, Malik RA et.al 2011 have done a observational study in Type 2 diabetic patients and found out that painful symptoms in 26% of patients without neuropathy and severe neuropathy in 60% of patients. [11] DPN affects nearly 50% of patient with diabetes mellitus and it is the commonest cause of complications in foot (ulceration, foot deformities, amputation, disability in ambulation). Various small trials were done in India, to known the status and to screen the incidence of DPN. The conclusion of majority of studies was an overall prevalence of neuropathy was 19.1% in type 2 diabetes mellitus patient of south Indian. Abbott CA, Malik RA 2011 has done A prospective study in older people with toe deformity, severe bunion, ulcer, and deformed nails, these patient with above problem had a two-fold increased risk of falling when compared with the healthy persons. Approximately 11-20% of falls may result in fractures.[12] Definition, Stages and Classification of Diabetic Neuropathy: Clinical Neuropathy: clinical signs and symptoms of neuropathy plus confirmation by abnormal quantitative neurological function tests (e.g., electrophysiological test, sensory testing, or autonomic function tests). Subclinical Neuropathy: it is defined as abnormal quantitative neurological function test predominantly sensory, with mild clinical evidence or no evidence of clinical neuropathy on examination. Clinical criteria and staging of diabetic neuropathy by Mayo Clinic: Stage Features Symptoms and signs Sensory tests (quantitative) abnormal 0 No neuropathy NO NO 1 Subclinical Neuropathy No Yes 2 Clinically evident Neuropathy Yes Yes 3 Debilitating Neuropathy Yes Yes Classification of diabetic neuropathy: 1. Symmetrical distal neuropathy 2. Symmetrical proximal neuropathy 3. Asymmetrical proximal neuropathy 4. Asymmetrical neuropathy and symmetrical distal neuropathy Clinical presentation: Numbness, Tingling, J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2010 ORIGINAL ARTICLE Pain (toes and soles of the feet, ankles, and lower shins) Sensory symptoms are usually worse at night when the patient is trying to sleep. Often, patients with diabetic neuropathy state that movement, walking, or standing lessens the pain. Types of pain: Dysesthetic pain: there is excessive firing in the nociceptive fibers which are damaged and particularly in the sprouting regenerating fibers. Paraesthetic pain: occur from various possible etiologies, (I) in the cell body of damaged afferent axons of the dorsal root ganglion there is increase spontaneous and mechano-sensitive activity (II) In the small unmyelinated fibers there is loss of segmental inhibition of large myelinated fibers. (III) Ectopic impulses produced from demyelination patches of myelinated axons. Muscular pain: it is due to ectopic impulses to muscles which is produced from demyelinated patches of motor nerves.[13] Motor signs and symptoms: Patient are not able to maintain their balance while walking, it is described as a swaying. They may also have ataxic gait during night time due to loss of joint and position sense. Leg weakness occurs lately in DPN, as patients usually do not complain of toe weakness, the first body region to be affected by weakness in most people with diabetic neuropathy. Autonomic Neuropathy: Autonomic neuropathies is a collection of syndromes and diseases affecting the autonomic neurons, either parasympathetic or sympathetic, or both. Autonomic neuropathies can be acquired or hereditary or acquired. Mostly occur in conjunction with somatic neuropathy, but they can occur in isolation also. Autonomic neuropathy may involve the cardiovascular system, gastrointestinal system, and genitourinary system and sweat glands. Patients with generalized autonomic neuropathies may present with ataxia, gait instability, or near syncope/syncope. In addition, autonomic neuropathies may have further symptoms that relate to the anatomic site of nerve damage gastrointestinal, cardiovascular, bladder, or sudomotor. Symptoms and signs: dry skin, cracked and mottled skin, giddiness, syncope, and abdominal bloating, altered bowel movements (constipation or diarrhoea), urinary incontinence sometime patient may develop retention of urine, and ejaculation impairment, Persistent sinus tachycardia, Orthostatic hypotension, Heat intolerance, sweating of head, neck, and trunk with anhidrosis of lower trunk and extremities. Numerous studies have shown that diabetic patient is more prone to have resting tachycardia and myocardial infarction predominantly silent in nature. [13] J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2011 ORIGINAL ARTICLE Neurologic examination: Physical examination begins with vital signs, pulse rate, measurement of blood pressure in sitting, supine, standing and lying position to assess changes in pulse and pressure alteration. In Patients with symptoms suggestive of orthostatic hypotension the measurements should be delayed for 1tio 2 minute after change in position. In normal cardiac function patients there should have an increase of pulse rate of 20-30 beats when there is change in position. Patients who are having crack in skin, dry skin, discourisation of skin, and changes in nails may have autonomic neuropathy. In mild diabetic distal neuropathy, there is reduction or loss of ankle reflexes and a gradient of a distal loss of small and large sensory fiber or "stocking-and-gloves" sensory loss. Examination of sensation of vibration using 128 Hz tuning fork is to check the presence or absence of vibratory senses at feet.[13] Monofilament testing: can assess by Semmes-Weinstein monofilaments (SWM). They are made of fine nylon, single-fiber nylon threads, identified by values ranging from 1.65 to 6.65, that generate a reproducible buckling stress.[14] In examination of the foot, various series of monofilaments fibres are used which ranges from 2.83 to 6.65. The monofilament tip is gently placed perpendicularly on the surface till the monofilament buckles. Pham et al., multicentric trial study on 248 patients found that 29% of patients (73 pts) or 19% i.e. (95 feet) developed foot ulcers.[15] Deep tendon reflexes: typically in neuropathy the ankle reflexes are first to lost followed by knee reflex. Upper limb reflexes are usually preserved in early diabetic neuropathy. Motor function: Weakness detected in toe extensors followed then toe flexors (length dependent nature of diabetic neuropathy). Proximal muscles of the legs are often spared, as the neuropathy progress to the knee, patients develop weakness of hand. Usually the weakness starts in the lower limbs in dorsal and ventral interossei muscles and abductor digiti minimi. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2012 ORIGINAL ARTICLE Electrophysiological testing: helps in evaluating patients with DSP by motor and sensory nerve conduction studies.[16] DPN is a significant cause in initiation of the pathophysiological pathway to ulceration of foot and amputation. Electrophysiological testing defines the nature of fibers which are affected in DPN (motor and sensory), but it also renders a gross estimation in the duration of the neuropathy, and even gives an insight into the prognosis.[16] Nerve conduction studies include: (I) Motor function evaluation of the median nerve, ulnar nerve, tibial nerves, peroneal nerve. (II) Sensory function of ulnar nerve, medial nerve, radial nerve, and sural nerve. (III) Velocities are termed in meters/second, motor amplitudes in mill volts. (IV) Sensory amplitudes in microvolt’s. Motor and sensory nerve functions of upper limb and lower limb helps us to show the distribution, presence, and severity of nerve disease. Nerve conduction studies (NCS): Gold standard for diagnosis of neuropathy Correlate with clinical scores, degree of nerve fiber loss is reflected with nerve amplitude In type 2 diabetic patients the abnormalities was detected in 40 to 60 %. Abnormality of NCS increases with the duration of diabetes. Motor nerve conduction study (MNCV) is an independent predictor in diabetic patients for the new foot ulcers. MNCV is used as a "benchmark" for distal symmetrical diabetic polyneuropathy assessment. Tibial nerve conduction: Tibal nerve is affected along with peroneal nerve, isolated damage of tibial nerve is very uncommon. Tibial nerve neuropathy results in the weakness of invertors, plantar flexors, and intrinsic foot muscles. Tibial nerve conduction study: electrode is placed on abductor halluces and slightly below to the anteriorly towards the navicular tuberosity, behind and proximal to the medial malleolus and in the popliteal fossa the stimulation is given. The tibial nerve conduction velocity in Indian population is 48.1 ± 4.6 μ/s. Common peroneal nerve conduction: axonal degeneration is the most common lesion irrespective of etiology of common peroneal neuropathy. Misra and Kalita et al suggested that the peroneal MNCV from tibialis anterior is the single most important electrophysiological finding. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2013 ORIGINAL ARTICLE Sural nerve conduction: patient having baker cyst are prone to have Compression injury to sural nerve. It is often involved distally in DPN. In metabolic neuropathy the Nerve conduction velocity get slowed down. Quantitative sensory testing: it is an essential method for quantifying sensory function in patients with polyneuropathies and specifically in DPN. QST have measurement errors as high as 30% when compared to amplitude measures in NCS.[17] Vibration perception threshold (VPT) is simple, inexpensive device, which is comparable to those obtained using more elaborate equipment and testing methods.[17] Thermal threshold test: it is less reliable compared to VPT test. Variation of 50% and higher is seen in thermal thresholds, due to high variation it has a limiting diagnostic value. Limitation of QST: it relies on subjective responses by the patient.[17] Clinical scoring scales: Various Neuropathy impairment scales are available for assessment of diabetic neuropathy which includes Nerve Conduction Studies, vibration perception thresholds, and autonomic function (heart rate with deep breathing). Drawbacks are i) time consuming, ii) overtly detailed and very difficult to inculcate in day-day practice in a clinical scenario. Michigan Neuropathy Screening Instrument (MNSI): it includes a questionnaire of 15items and feet clinical examination. Michigan diabetic neuropathy score (MDNS): it is a clinical score of 46 point which was developed by Feldman et al. It has two parts, Examination of Central nervous system and nerve conduction.[18] Feldman et al. compared the scores of MDNS & MNSI. MDNS with NCS helped in confirming the diagnosis, and MNSI helped in good screening tool.[18] Posturography: Postural stability is usually defined as the ability to modify postural strategies in response to changing surface and environmental demands. This response is impaired in DPN leading to higher risks of fall.[19] Ahmmed et al., concluded that patients with DPN had more postural sway as compared to patients with diabetes mellitus without complication and healthy controls.[20] Postural stability can be evaluated in terms of velocity of sway, moment velocity, and length of sway, under four conditions like: Eyes open (EO), eyes closed (EC), eyes open on foam (EOF), and eyes closed on foam (ECF). Lafond et al., reported that there is impairment in the static stance in DPN.[21] CONCLUSION: In type 2 diabetic patients measurement of Peripheral nerve function is done through clinical approach, investigation approach, and this can help us to evaluate the severity of nerve function and those who are predispose to develop the foot deformality, ulceration. Patients with type 2 diabetes are prone to develop DPN. It carries risk of pain and trophic changes and autonomic dysfunction. All the treatment given to DPN is good glycemic control, pain inhibitor, but no 100% cure remedy is available for the treatment of DPN. Once neuropathy J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2014 ORIGINAL ARTICLE is diagnosed, patient should be educated regarding the complication like tropic ulcers, deformalites, if neglected may result in disabling motor and sensory deficit or may lead to amputation. Routine clinical evaluation and performing these tests can prevent the patient from complication. REFERENCES: 1. Seshiah V. Diabetic Neuropathy. In: Balaji V, Patel M, editors. Handbook of Diabetes Mellitus, 4 th ed. New Delhi: All India Publishers; 2009. p. 188-97. 2. Gimeno SG, Ferreira SR, Franco LJ, Hirai AT, Matsumura L, Moises RS. Prevalence and 7year incidence of Type II diabetes mellitus in a Japanese-Brazilian population: An alarming public health problem. Diabetologia 2002; 45: 1635-8. 3. Martin CL et al. (2006) Neuropathy among the Diabetes Control and Complications Trial Cohort 8 years after trial completion. Diabetes Care 29: 340-344. 4. Palumbo PJ et al. (1978) Neurologic complications of diabetic mellitus: transient ischemic attack, stroke and peripheral neuropathy. In Advances in Neurology, vol 19, 593-601 (Ed Schoenberg BS) New York: Raven Press. 5. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4-14. 6. Misra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidemia in urban slum population in northern India. Int J Obes Relat Metab Disord 2001; 25: 1722-9. 7. Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and glucose tolerance in India. National Urban Diabetes Survey. Diabetologia 2001; 44: 1094-101. 8. Narayan KM, Echouffo-Tcheugui JB, Mohan V, Ali MK. Analysis and commentary: Global prevention and control of type 2 diabetes will require paradigm shifts in policies within and among countries. Health Aff 2012; 31: 84-2. 9. Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms, 2 nd ed. Seattle: IASP Press; 1994. p. 209-14. 10. Tesfaye S, Vileikyte L, Rayman G, Sindrup S, Perkins B, Baconja M, et al. On behalf of the Toronto Expert Panel on Diabetic Neuropathy. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011. 11. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011; 34: 2220-4. 12. A Global Report on Falls Prevention Epidemiology of fall. WHO Report. Available from URL: http://www.who.int/ageing/publications/Falls_prevention7March.pdf [Last accessed on 2013 Feb 4. 13. Tanenberg RJ, Donofrio PD. Neuropathic problems of lower limbs in diabetic patients. In: Bowker JH, Pfeifer MA, editors. The Diabetic Foot. 7 th ed. Philadelphia: Mosby Elsevier; 2008. p. 33-40. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2015 ORIGINAL ARTICLE 14. Dros J, Wewerinke A, Bindels PJ, van Weert HC. Accuracy of monofilament testing to diagnose peripheral neuropathy: A systematic review. Ann Fam Med 2009; 7: 555-8. 15. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration. Diabetes Care 2000; 23: 606-11. 16. Perkins BA, Bril V. Diabetic neuropathy: A review emphasizing diagnostic methods. Clin Neurophysiol 2003; 114: 1167-75. 17. Gelber DA, Pfeifer MA, Broadstone VL, Munster EW, Peterson M, Arezzo JC, et al. Components of variance for vibratory and thermal threshold testing in normal and diabetic subjects. J Diabetes Complications 1995; 9: 170-6. 18. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281-9. 19. Corriveau H, Prince F, Hebert R, Raiche M, Tessier D, Maheux P, et al. Evaluation of postural stability in elderly with diabetic neuropathy. Diabetes Care 2000; 23: 1187-91. 20. Ahmmed AU, Mackenzie J. Posture changes in diabetes mellitus. J Laryngol Otol 2003; 117: 358-64. 21. Lafond D, Corriveau H, Prince F. Postural control mechanisms during quiet standing in patients with diabetic sensory neuropathy. Diabetes Care 2004; 27: 173-8. AUTHORS: 1. Riyaz Mohammed PARTICULARS OF CONTRIBUTORS: 1. Associate Professor, Department of General Medicine, MNR Medical College. NAME ADDRESS EMAIL ID OF THE CORRESPONDING AUTHOR: Dr. Riyaz Mohammed, 11-5-337/2 Redhills, Hyderabad, Telangana – 500004. E-mail: riyazesani@hotmail.com Date Date Date Date of of of of Submission: 02/12/2014. Peer Review: 03/12/2014. Acceptance: 12/12/2014. Publishing: 18/12/2014. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/Issue 16/Dec 22, 2014 Page 2016