Guidance for the choice of outcome measures
advertisement

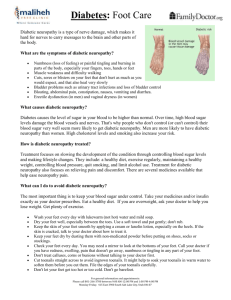
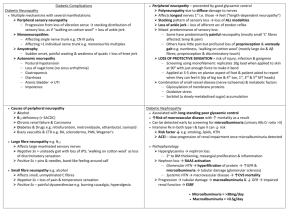
Cochrane neuropathy outcome measures 15.7.04 Guidance for the choice of outcome measures for Cochrane reviews of interventions for peripheral neuropathy with special reference to diabetic neuropathy 1. Each review should pre-specify in its protocol one primary outcome measure at one time or for one period. Alternatively where results have been expressed as hazard ratios at different times, possibly obtained from differing periods of observation, the survival curves may be combined as described by Parmar1 or the hazard ratios combined using the Generic Inverse Variance weighted average facility in RevMan. The same outcome for different times or periods or as an average rate of change from several time points may be included as secondary outcomes. 2. The primary outcome may be selected by the authors of each review and may differ from review to review. 3. The primary outcome in any existing protocol or review should be included as a secondary outcome in all other reviews of interventions for diabetic neuropathy. 4. It is usual to have up to five secondary outcome measures of which one must be the occurrence of adverse events in a specified period, usually the period during which the intervention was applied. Since randomised trials rarely contain adequate information about adverse events, authors should stipulate that they will also seek evidence of adverse events from non-randomised information and report this in the discussion. 5. In other conditions, the Cochrane Neuromuscular Disease Group prefers measurement of disability (otherwise known as “activities” in the most recent WHO classification) as having immediate and obvious relevance to patients. In diabetic neuropathy, it is unlikely that disability measures will have been collected or measured during a trial. 6. Important ultimate outcomes would be the occurrence of foot ulcers or amputation but the size of the trials needed to permit detection in changes in this outcome may make the choice of this outcome impractical. 7. For trials of interventions to treat symptoms, especially pain, a symptom score is appropriate. Ideally scores should be biometrically sound with known face validity, reproducibility and responsiveness. Results should be always be interpreted in the context of the quality of the measure used. 8. Measures of quality of life would have obvious relevance to patients and should be included when possible. Unfortunately, they have not been widely used in treatment trials for diabetic or other neuropathy. 9. Measures of impairment may be made from the neurological examination aided by quantitative tests of muscle strength or sensory function. Tests of similar functions such as a clinical sensory sum score and a quantitative test of sensation could be combined in a metaanalysis. 10. Neurophysiological measures should be considered separately from clinical measures of impairment. Scores of sensory nerve conduction tests should be considered separately from those of motor nerve conduction tests. 11. The above statements challenge the use of composite scores in meta-analyses. This should only be done when more than one trial has used the same composite score. 12. Where different scales measuring the same attribute have been used, meta-analysis can be performed in two different ways: a. by using standard deviations as the units and reporting standardised mean differences:. Consideration must be given as to which standard deviation to choose and in general it should be the standard deviation of the attribute in the population being tested at baseline. or b. by dichotomising changes into ‘clinically significant improvement’ or not. The metaanalysis can then be used to pool the relative rates of improvement comparisons. If a method of defining clinically significant is not obvious then computing the proportion that improve by an arbitrary amount such as half a standard deviation may be appropriate. Richard Hughes, Tony Swan, David Cornblath 15 July 2004 1. Parmar KB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998; 2815-2834. This is also Cochrane neuropathy outcome measures 15.7.04 discussed in the statistical section of the Cochrane Neuromuscular Disease Group Module in the Cochrane Library.