seizure pathphys
advertisement

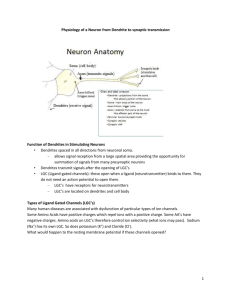
Pathophys of seizures Basic Physiology o In the cortex there are 2 types of neurons: [very simplified] Principle neurons – ones that project and send messages to distant parts of brain Interneurons – local neurons with generally inhibitory influence Important to note that these type of neurons can act on neurons of the same type or the other type o Eg. Hyperactive inhibition of an interneuron on an interneuron leading to a principle neuron may lead to an overall effect of hyperstimulation. o Two major classes of neurotransmitters: Stimulation : Glutamate Postsynaptically on both types of neurons! Receptors: AMCA, kainate, NMDA – these receptors differ from eachother in levels of permeability and in therapeutic targeting. Allow ion influx into nerve. All channels permeable to Na+K, AP What makes the NMDA glutamate receptor special? o They have their own calcium channels, that normally are blocked by Mg ions, but when they want to become activated, they drop the Mg, and let Ca into the cell, which causes heaps of depolarization, making these receptors very important! However, too much Ca can kill the cell! As a general rule: Agonise these receptors, seizure inducing, Antagonise, seizure suppression Inhibition: GABA Receptors: GABA-A and GABA-B: o A is post-synaptic and B is pre-synaptic (modulate synpatic release) A receptors are very permeable to chloride ions, so when they become activated, theres a Cl influx, they hyperpolarise the cell, leading to reduced action potentials, leading to increased neuronal activity. Agonise these A receptors and you will reduce seizure activity. B receptors: less relevant for seizure therapy; but these guys rely on secondary messenger activation (which activates their K ch’s., rather than chlorine. When they become activated, they can attenuate transmitter release (remember their pre-synaptic location) What modulates neuronal excitation? Neuronal factors: Internal The type, number and distribution of voltageand ligand-gated channels. Biochemical modification of receptors Activation of second-messenger systems Modulating gene expression External Changes in extracellular ion concentration due to variations in the volume of the extracellular space Remodeling of synaptic contacts Modulating transmitter metabolism by glial cells Networking factors: o The way the nerves are connected can alter potential excitation. Inhibitory vs excitatory neuronal influences on eachother and themselves. Pathophysiology May start in a focal area of the brain and spread or may actually spontaneously occur widespread thoughout different areas of the cortex. This, as well as the site of these occurrences, has a far greater influence on the clinical presentation than the actual molecular patholophysiologcal basis of the seizure. Generalised vs. Focal. Acquired epilepsy: Epileptogenesis: o ~50% of patients of who suffer severe brain trauma will develop a seizure disorder. o There is a latency period (often of a couple of years) between these two events o This is the gradual transformation of the normal brain to the hyperexcitable brain o Delayed necrosis of inhibitory neurons (or their feeding stimulating ones) o Sprouting of axonal collaterals leading to reverberating circuits. 1. High-frequency AP bursts The synchronized bursts from a sufficient number of neurons result in a so-called spike discharge on the EEG. Sustained neuronal depolarization resulting in a burst of action potentials, a plateau-like depolarization associated with completion of the action potential burst, and then a rapid repolarization followed by hyperpolarization. o This sequence is called the paroxysmal depolarizing shift. The bursting activity resulting from the relatively prolonged depolarization of the neuronal membrane is due to influx of extracellular Ca++, which leads to the opening of voltagedependent Na+ channels, influx of Na+, and generation of repetitive action potentials. The subsequent hyperpolarizing afterpotential is mediated by GABA receptors and Cl− influx, or by K+ efflux, depending on the cell type. 2. Hypersynchnoisation of neurons Seizure propagation, the process by which a partial seizure spreads within the brain, occurs when there is sufficient activation to recruit surrounding neurons. This leads to a loss of surround inhibition and spread of seizure activity into contiguous areas via local cortical connections, and to more distant areas via long association pathways such as the corpus callosum. The propagation of bursting activity is normally prevented by intact hyperpolarization and a region of surrounding inhibition created by inhibitory neurons. With sufficient activation there is a recruitment of surrounding neurons via a number of mechanisms. Repetitive discharges lead to: 1) an increase in extracellular K+, which blunts the extent of hyperpolarizing outward K+ currents, tending to depolarize neighbouring neurons; 2) accumulation of Ca++ in presynaptic terminals, leading to enhanced neurotransmitter release; and 3) depolarization-induced activation of the NMDA subtype of the excitatory amino acid receptor, which causes more Ca++ influx and neuronal activation. A lot less is known about spontaneous cessation of seizures, status epilepticus is where this does not occur