Biomedical Waste - Linda Rojas Knight
advertisement

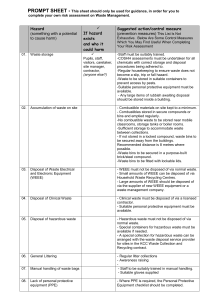
Biomedical Waste Safety & Regulatory Compliance VETE 411-011 Linda R Knight March 6, 2015 Proper disposal of waste may appear straightforward at first, but differences in handling an item may contribute to factors that alter its waste stream- that is, the process by which it’s disposed of. Waste, is defined as everything that no longer has a use or purpose and needs to be disposed. The term surely applies to discarded material, but there are precise classifications for waste that affect how waste is regulated and must be handled, particularly in professional settings. The majority of veterinary practice waste is considered "solid waste," regardless of whether it's actually "solid" in physical form. Solid waste created by veterinary clinics include, but aren't limited to: animal tissues, fluids and carcasses; animal waste; chemicals used in laboratory, cleaning or radiographic procedures; syringes and other medical supply waste; some medications, such as epinephrine and nitroglycerin; chemotherapy agents; mercury from thermometers; light bulbs and batteries; pesticides; paint and other solvents (AVMA, 2015). For instance, the proper removal of a portion of intravenous tubing used to deliver a chemotherapeutic drug to a patient can be important simply by flushing the IV line during chemotherapy. However some key points that we can install and/or police disposal choices include (AVMA, 2015): Awareness of the options and restrictions for disposal of individual items in your area Knowing which authorities have oversight over which aspects and items so that you know who to ask when questions come up Disposal instructions on Material Safety Data Sheets (MSDS) and product inserts Disposal policy for the practice including all of the above Training Due to the diverse nature of the products that are disposed of in veterinary practices, more than one federal agency is involved in regulating the disposal progression. Simply put, the Biomedical Waste | Safety & Regulatory Compliance VETE 411-011 Page 1 of 3 Environmental Protection Agency (EPA) regulates the disposal of products with environmental impact; the Occupational Safety and Health Administration (OSHA) regulates factors associated with possible employee exposure to hazardous materials; the National Institute of Occupational Safety and Health (NIOSH) offers directions pertaining to products used in the workplace that impact human and public health; and the Drug Enforcement Agency (DEA) regulates the disposal of controlled substances (AVMA, 2015). Furthermore, veterinarians are often not aware that they are subject to regulations by federal agencies such as Department of Transportation (DOT, and Nuclear Regulatory Commission (NRC). Likewise, veterinarians are subject to regulations if they ship hazardous materials whether it be chemicals or laboratory specimens. For instance, if veterinary practices ship via ground, they are subject to DOT, but shipping via air they are subject to FAA regulation. Clinics using nuclear scinitgraphy or radionuclide therapy, are subject to NRC (EPA, 2012). In any given state, EPA or State hazardous waste regulatory agency imposes waste laws that all institutions must adhere. Hazardous waste is unwanted properties that make it dangerous or possibly unsafe to human health or the environment. Federal agencies such as, EPA encourages States to accept responsibility for employing a hazardous waste program through acceptance, approval, and implementation of the rules. Biomedical Waste | Safety & Regulatory Compliance VETE 411-011 Page 2 of 3 Reference: Veterinary Practice Waste Disposal Decision Maker, 2015. American Veterinary Medical Association, Retrieved March 5, 2015 https://www.avma.org/PracticeManagement/Administration/Pages/Veterinary-Practice-WasteDisposal-Decision-Maker.aspx Hazardous Waste Regulations, 2012. U.S. Environmental Protection Agency. Retrieved March 6, 2015 http://www.epa.gov/osw/laws-regs/regs-haz.htm Biomedical Waste | Safety & Regulatory Compliance VETE 411-011 Page 3 of 3