Rutherford Scattering Activity
advertisement

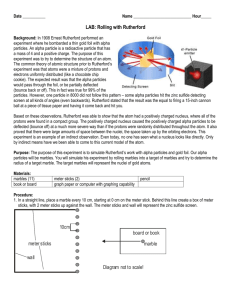
Rutherford Scattering: Atomic Target Practice Background: Ernest Rutherford received a Nobel Prize for his work with radioactive decay. When an element decays, it gives off alpha particles. Alpha particles are small, positively charged and have a lot of energy! Rutherford decided to test what would happen when he directed a narrow beam of alpha particles at different types of metal foil. He expected that the particles, having so much energy, would simply pass through the foil and be measured on the other side. What was unexpected was that a few particles – very few- bounced back! Somehow, the particles were reflected back. More interesting was that if they used a metal with a larger atomic mass, more particles would be reflected! Gold metal, with the highest atomic mass, gave the largest amount of particles that were reflected back. At the time, scientists understood alpha particles. They knew their speed, mass, and charge. However, scientists did not understand atoms, like the ones that made up the metal pieces. When an experiment is done on an unknown object, it may be referred to as a “black box” experiment. This is because one of the elements is mysterious or unknown, almost as if it were hidden away in a black box. Rutherford was able to explain the results of his experiment only by unraveling the mystery of the atomic black box. In 1911, he proposed these ideas about an atom, in order to explain what happened in his famous experiment. Most of the mass of an atom is in a small, dense, center (now called the Nucleus). The center of the atom is positively charged. Most of the atom is empty space! Experiment Overview: The purpose of this activity is to discover by indirect means the shape and size of an unknown object, which is hidden beneath the middle of a large board. By observing and tracing the path of a marble rolled underneath the board, it should be possible to estimate the general size and shape of the unknown target. For your lab report: Meaningful title, a 1-2 sentence purpose statement, and materials list Answer the pre-lab questions. Write a concise procedure. Write a conclusion paragraph Materials: “Black box” Marble White paper (2 pieces) Tape Pencil Pre-lab Questions: 1. In Rutherford’s lab, alpha particles were shot toward metal foil, and bounced off the nucleus. In our lab, what is like the alpha particles? What is like the metal foil? What is like the nucleus? 2. It is important to trace the path of the marble roll, even when the marble rolls straight through without striking the unknown target. What information can be inferred based on where the marble rolls straight through? Rutherford Scattering: Atomic Target Practice 3. Assume the marble hits the following sides of a possible target. What would you expect the path of the marble to be in each case? marble marble marble Procedure: SPECIAL NOTE: In order to obtain good results for this experiment, it is very important that straight and accurate lines and angles are drawn! The use of colored pencils or different pens is highly recommended to allow you to keep track of which lines are which! 1. When you arrive at the lab station, you will find a piece of cardboard taped to the top of a hidden object. DO NOT LIFT UP THE CARDBOARD OR LOOK UNDERNEATH IT! 2. Place a sheet of paper on top of the cardboard and tape its corners in place. WRITE ONLY ON THE PAPER, NOT THE CARDBOARD! 3. Using a ruler as a guide, roll the marble under the cardboard with moderate force (too light and it will not have enough energy to bounce off the shape if it hits it, too strong and it will be hazardous to others as it flies off the table top.). Draw a line on the paper to show the path of the marble as it entered and exited. 4. Repeat step #3 from all sides until you have enough lines to determine the shape of the object underneath. 5. When you think you have determined the shape of the object, have your drawing checked by your instructor before removing the paper from the cardboard. Data Analysis: Each lab group should label the drawing(s) obtained during this lab on the back with the names of the students participating, the class hour, and the object letter. These are to be turned in at the end of the hour. Conclusion: o In what 2 ways does this lab simulate Rutherford’s efforts to determine the structure of the atom? In what 2 ways is it different? o You had the satisfaction of eventually seeing the shape under the cardboard. Did the early atomic scientists have the same opportunity? Would it have affected your enjoyment of the lab if you had never been told whether you had determined the shape correctly? Why or why not? o Design another simulation or game that illustrates the problem of identifying something without being able to observe it directly.