Lab report 2 - WordPress.com
advertisement

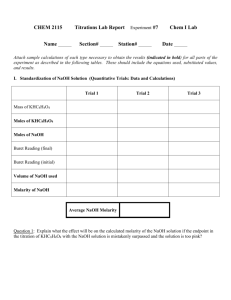
Maram Alfi, Salma Omar, & Kayla Phillips March, 2, 2010 General Chemistry 110 lab Yumi Matsumura The best Antacid that can neutralize the HCl using “Titration” Abstract This experiment is about to decide which antacid is the best to make the HCl neutralized. There were two antacids that were provided which are: CVS antacid tablet and a Brioschi tablet. These two tablets were used in order to neutralize the HCl. The method used was titration. Titration is basically using an unknown solution with a known solution (What’s the best Antacid?). The results were that the CVS antacid is better to use based on the price. It cost $.02 per dose. Some antacids are more effective than others and that is what showed when two antacids were used. The CVS antacid is more effective to neutralize the HCl and easy to use. Introduction There are many antacids in different shapes of matter. It can be solid or liquid. Antacids are different in the how much each antacid can neutralize the HCl. This experiment is about comparing two antacids and knowing which one is the best to neutralize the HCl. The method that is used to know which antacid is the best is titration. Titration was used in this experiment with the two chosen antacid to see which one is the best antacid. Titration was about putting HCl in a tube and then, after adding the antacid to the water, the baker was put under the tube that had the HCl. The HCl was added to the water and the antacid in the baker into doses. Slowly adding HCl until the water with the antacid turns purple pink. The purpose of this experiment is to decide which antacid is the best to neutralize the HCl. There are four equations that are used to help determine the dose that should be used with the right amount of HCl. EQ 1 Moles HClinitial = Molarity HCl X 1L X mL of HCl use 1000 ml EQ 2 Moles HClNaOH neutralized = M of NaOH X 1 L X mL NaOH used X 1 mol HCl 1000 mL EQ 3 1 mol NaOH Moles of HClantacid neutralized = (moles of HClinitial) - (moles of HClNaOH neutralized) EQ 4 Moles of HClantacid neutralized/gram = Moles of HClantacid neutralized/grams antacid used EQ 5 Moles of HClCaCO3 neutralized per dose = (550 mg CaCO3) * (1 g/1000 mg) *(1 mol/100.09 g) * (2 mol HCl/1 mol CaCO3) = 0.0101 mol HCl (What’s the best Antacid?). Experimental Method The equipments for this experiment were: bakers, tube, holder, HCl, NaHCO3, two different types of antacid, pipette pumps, heater, and phenolphthalein. First, the tube was full of NaHCO3. Meanwhile, the baker was full of water till 75 mc in the baker and the antacid was broken to the amount required based on the calculations. The first antacid was the CVS antacid tablet. After the water has been added to the baker, and two drops of phenolphthalein were added too using volumetric pipettes, the antacid was added based on the requirement quantity which was 6%. After that, the antacid was heated till it boils for 2 minutes. The antacid has been stirred. The NaHCO3 has been added to the antacid after stirring it and the NaHCO3 was added every 30 seconds till the color turned purple pink. These steps were used to the other antacid, Brioschi tablet, following the same steps. Results The antacids that were used based on required quantities. The following table shows the types of the antacids that were used and the specific amount of each. The antacids Brand names Tablet or liquid Tablet to be crushed (Y/N) Acid/Antacid mixture to be boiled An amount of antacid to be used Active Ingredient(s) grams/dose Antacid cost per container Antacid 1 Antacid 2 CVS antacid Brioschi Tablet Tablet Y Y Antacid Antacid 6% .14% CaCo3 NaHCO3 3.29 8.49 Size of container (#tablets or mL) 150 Tablets 240g $ .02 $ .06 per dose Cost per dose Following the equations that are mentioned above, these equations were used to choose the right amount of each antacid that has been used. For the CVS antacid Equation 5 Moles of HClCaCO3 neutralized per dose = (500 mg CaCO3) * (1 g/1000 mg) *(1 mol/100.09 g) * (2 mol HCl/1 mol CaCO3) = 0.099 mol HCl .006/.099 =.06 the percentage of how much was used from the CVS antacid When the NaHCO3 was added to the antacid after boiling, the amount of how much NaCOH3 was measured to know how much was used to make the antacid purple pink. For the CVS antacid, the NaHCO3 started with 7 ml and ended at 50 ml = 43 ml. Discussion The purpose of this experiment is to know what the best antacid is to neutralize the HCl. As mentioned, two antacids were used in this experiment. Both of these antacids were used with a specific amount based on the calculations and the equations. Following the steps and after heating the antacids, it has been noticed that the two antacids were similar in how much it took to boil and also to turn to a purple pink color. At the start of doing the experiment, the calculations were needed to decide how much should be used of each of the antacid, such as the calculation of the CVS antacid, Moles of HClCaCO3 neutralized per dose = (500 mg CaCO3) * (1 g/1000 mg) *(1 mol/100.09 g) * (2 mol HCl/1 mol CaCO3) = 0.099 mol HCl The calculations were helpful because it was needed to know how much to use and not choose random amounts which may lead to different results. Moreover, when the NaHCO3 was added to the two antacids, the both antacids took almost the same amount of NaHCO3 and time to change to a purple pink color. That was clear when the calculations were done. The CVS antacid started at 7 ml and ended at 50 ml which equals 43 ml. The Brioschi antacid started at 5 ml and ended at 48 ml which equals 43 ml. If there was a mistaken amount in any of these elements, the results will be different. There can be errors when adding more than two drops of phenolphthalein or use more than the amount required of the antacid. Moreover, the errors can be in how to add the liquids in the baker using the pipette pump. Using the pipette pumps should be in a carful way because not being careful may cause the damage for the baker and the pipette pumps. The best way to prevent errors is to ask for help for the teacher assistant or the professor and follow the steps carefully and understand them. Reference Blackboard document What’s the best Antacid?. ( 2010). Chemistry lab.