PennyBattery v2 - Discover Technology
advertisement

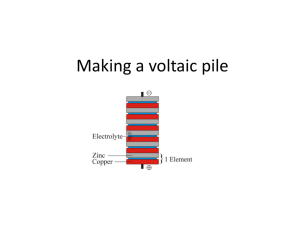
The Penny Battery Overview Transform pennies and zinc washers into a battery that will power a light emitting diode (LED). Next Generation Science Standards ● NGSS MS-PS1-6 The transfer of energy can be tracked as energy flows through a designed or natural system.http://www.nap.edu/openbook.php?record_id=13165&page=94 ● NGSS MS-PS3-2 Develop a model to describe that when the arrangement of objects interacting at a distance changes, different amounts of potential energy are stored in the system. Key Concepts Light emitting diode (LED) Chemical energy Batteries in series Voltage Multimeter use Materials ● 3 Pennies – Pennies from before 1982 work best because they are 95% copper; those made after 1982 are 97.5% zinc with a thin copper coating. Pennies made in 1982 could be either zinc or copper. ● Cardboard ● Vinegar ● Scissors ● Plastic cup ● 3 5/16 inch zinc flat washers ● Red LED (available from Radio Shack) ● Electrical tape ● Multimeter ● 1 in by 3 in piece of aluminum foil Duration 30 minutes Grade Level 4-9 Safety Concerns Vinegar is an irritant. Wear safety goggles. Procedure 1. Trace around 3 pennies that have been placed on cardboard. 2. Cut the cardboard pieces so they are slightly bigger than a penny. 3. Soak the cardboard pieces in vinegar until they are saturated. 4. Place a zinc washer on one end of the aluminum foil then stack a piece of vinegar soaked cardboard followed by a penny. This is one cell of the battery. . Use a multimeter to measure the electric potential in volts by placing the positive (red) lead on the top penny while the black lead is placed on the aluminum foil under the stack. You can also measure current draw in milliamps. 5. Continue this pattern until the desired voltage is reached measuring the electric potential after each cell. 6. Connect the negative lead of the LED (short leg) to the zinc washer side of the battery and the positive side to the penny. 7. Wrap the entire battery with electrical tape. The LED should continue to shine. Extension The development of the wet cell battery has an interesting connection to biology. Alessandro Volta, who invented the battery or Voltaic pile in 1800, was said to be inspired by the torpedo fish. Capable of electrocuting its prey; the torpedo fish uses an organic structure to produce an electric shock. The fish has electrocytes which are flat disk-like cells that are stacked just like the copper and zinc disks in the battery created in this activity. The more cells that are stacked, the more the voltage produced. Investigate the connection between the study of the torpedo fish and the invention of the battery.