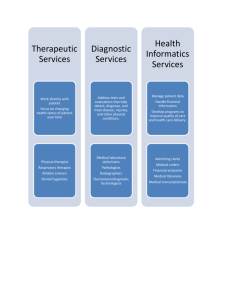
Laboratory techniques for technicians
advertisement

Laboratory Techniques for Environmental Technicians Environmental Monitoring & Technology Series Chemical, Forensic, Food & Environmental Technology Laboratory Techniques For Environmental Technicians Trainee Learner Resource Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] cffet.net/enviro Course Notes for delivery of MSS11 Sustainability Training Package Page | i Laboratory Techniques for Environmental Technicians Chapter 1: Introduction and Induction Practicals and theory Introductory Laboratory Practice - details and back-ground information Workplace protocols Chapter 2: The language and structure of Chemistry What is Chemistry? Practical: Observation Skills Classification schemes Practical work Practical: Physical versus chemical change Bohr model of the atom The Periodic Table & Atomic Structure Chemical Formulae Types of compounds Organic chemicals The Mole Concept Solutions Calculating the molarity of a solution Dilution of solutions Practical: Metal colours in a flame 4 4 4 5 6 6 6 7 12 17 19 27 28 31 38 43 47 48 50 52 Chapter 3: Safety in the Laboratory 53 Safety in the Laboratory Laboratory hazards Laboratory rules and regulations Practical 1.1 Laboratory Layout Clean-up of spills Practical 1.5 Safety in the laboratory Dangerous goods 'Class' labels 53 54 56 57 58 59 62 Chapter 4: Basic Laboratory Equipment Basic Laboratory Equipment Chapter 5: Introduction to Material Handling Material transfer Practical work 5.1 Basic material handling techniques Volume measurement techniques Accurate and Approximate Volume Measurement Pipetting Techniques Burette Techniques Volumetric Flask Techniques Measurement Of Mass Practical work 5.2A Introductory weighing task Other Basic Laboratory Procedures Practical work 5.5A Simple filtration Practical: 4.4 Heating devices 64 64 70 70 72 73 73 75 76 78 79 80 81 82 85 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | ii Laboratory Techniques for Environmental Technicians Practical: Application of heating equipment 87 Chapter 6 The dreaded calculations 89 Laboratory Data and Error Analysis Classification of Errors 89 90 Chapter 7: The why’s of sampling Practical: Practical: Validation of sampling Sampling Equipment Chapter 8: Solution Preparation Practical 5.4A Introductory Solution Preparation Chapter 9: Basic environmental laboratory testing Laboratory Measurements Practical work 7.1 pH measurements Measurement of density Practical: 7.7 Density Chapter 10: Gravimetric analysis Gravimetric Analysis Practical: Analysis of a mercury chloride and determination of its empirical formula. Practical: Gravimetric determination of sulfate in bore water 8.6 Practical: Experimental Investigation of combustion of magnesium Practical: Gravimetric determination of Ni by precipitation with dimethylglyoxime. Chapter 11: Volumetric Analysis Types of Standards used in Titration Titration Calculations Practical: Practice titration Practical: Ethanoic acid content of Vinegar Practical: Sodium Carbonate Content of Washing Soda Practical: Chloride by titration with silver nitrate Practical: Determination of hardness in water Chapter 12: The Open Ended Problem Open ended project 95 104 107 109 110 112 112 112 114 115 119 119 121 123 126 129 132 138 141 143 145 147 149 151 154 154 Assessment 1 156 Assignment 1 Assignment 2 Assignment 3 Assignment 4 156 157 158 159 Assessment 2 160 Assignment 1 160 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | iii Laboratory Techniques for Environmental Technicians Chapter 1: Introduction and Induction The Chemical, Forensics, Food & Environmental Technology (CFFET) section of Hunter TAFE has developed this manual to help online students in the area of Laboratory Practice. This manual is designed to be used in conjunction with the text “Practical Laboratory Skills” (1995 Harcourt Aust) by Krajniak, Barker & Fullick. It is not a requirement to purchase this text as the required information will be provided throughout the chapters that follow. The CFFET team gratefully acknowledge the permission of the authors to reproduce text and diagrams in these notes. Laboratory Practice is a general term used by this teaching section to describe standard operating procedures in a typical science laboratory. Activities in this manual have been designed to help candidates learn and practice skills necessary to be deemed competent in some of the competency standards described in the Laboratory Operations Training Package – MSL09. Laboratory Practice covers all the specified activities, defined in TAFENSW curriculum, to develop general science laboratory competencies. Practicals and theory This manual only provides brief introductory notes for each activity and it is expected that the teacher and the textbook will provide more extensive details of basic theory and the contexts in which the theory may be applied. The additional information will be provided during the intensive session. The practical manual provides result sheets to record and report the laboratory activities, observations and measurements made which summarise the learner’s work. These completed result sheets will become part of the evidence of the learner’s skills needed to assess competency. There will be selected activities or responses required prior to the intensive session these will be identified by a box requiring an answer that will be sent off to the trainer. Introductory Laboratory Practice - details and back-ground information Staff details: Head Teacher ………………….Dr. G. Fullick…………………………………… Class Teacher ………Adam Samuelson; Daniel Solomon; Denise Hatton Technical staff……depends on availability…………………… Specific details relating to working in the Science Building of Hunter Institute will be provided at the first session during the intensive session. The following page highlights the workplace protocols that apply to the Edgeworth David Building, Hunter Institute. These will be discussed in the induction first session Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 4 Laboratory Techniques for Environmental Technicians Workplace protocols 1. Safety Procedures (a) (b) (c) (d) (e) (f) (g) Consult Safety Data sheets* and method of analysis for advice on hazards and precautions to be taken Wear appropriate PPE Use fume hood etc as necessary Maintain tidy workspace Exercise care not to endanger other people Observe emergency procedures Report spillages and all accidents 4. Testing (a) Refer to workplace procedures manual for standard method Conduct tests according to workplace procedures Clean up spills promptly Record results according to workplace procedures, without alteration Calculate results, checking against expected values and correcting errors Trouble shoot basic problems with procedure or equipment which have led to atypical results (b) (c) (d) (e) (f) 2. Recording and Reporting (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) (o) Register samples into laboratory system Label samples Record which tests the sample should undergo Record sample description, compare with specification, record and report discrepancies Record calibration results for instruments/equipment in tables and/or charts, following quality system Keep records of calibration status and calibration schedule for instruments / equipment Report faulty equipment Keep records of solutions prepared, by expected use-by date, and by name of person who prepared them Record results legibly, and chart when required to identify trends Interpret trends Identify and report atypical results promptly to appropriate personnel Record approved results into workplace system Comply with quality system Report all accidents and potential hazards Maintain confidentiality of workplace information 3. Sample Handling (a) (b) Maintain sample integrity Prepare sample and standards for test 5. Equipment and Reagents (a) (b) (g) Set up equipment and reagents Check calibration status of equipment; calibrate if necessary Monitor shelf-life of working solutions Prepare solutions when necessary, label and log into laboratory register Clean and care for test equipment and work space Dispose of faulty equipment or quarantine it for repair Store unused reagents 6. Wastes (a) (b) Minimise generation of wastes Collect, sort and dispose of wastes in accordance with procedures 7. (a) Environmentally specific Equipment not in use is turned off at the power switch Lights are off when laboratory is unattended Fume cupboards are off when not in use Non-conformances with environmental standards are reported (c) (d) (e) (f) (b) (c) (d) * SDS’s were up until January 1 2012 referred to as MSDS (Material Safety Data Sheets) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 5 Laboratory Techniques for Environmental Technicians Chapter 2: The language and structure of Chemistry What is Chemistry? Scientists like to group the things they observe. It provides a way of organising and putting their observations in order. I has evolved over many thousands of years using the observation skills of many people. Both quantitative and qualitative information is important. What is the difference between the two terms: Quantitative information This is any information that has measurement attached to it. It could be a temperature measurement for example 25°C or a length for example 25 mm or the number of trees in a 100 m2 area is 11. Qualitative information This is information that has no exact amount associated. It is hot or it is cold or the frog is about 10 cm. Many important scientific discoveries have been the result of someone noticing something that many may not have observed or treated as insignificant. It is important to develop both your quantitative and qualitative observation skills. Observation skills are particularly important in aspects of laboratory work. We use all senses to assist in working safely and also in noting aspects of our practical work Practical: Observation Skills Take a candle and: Make as many observations as you can about your candle. Type you answer here Leave blank for assessor feedback Light your candle and record all your observations Type you answer here Leave blank for assessor feedback Blow out your candle and record your observations Type you answer here Leave blank for assessor feedback Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 6 Laboratory Techniques for Environmental Technicians Classification schemes List any classification systems for science with which you are familiar. Type you answer here Leave blank for assessor feedback Matter is a good starting point for classification and hence the study of chemistry. Matter, Change and Energy Chemistry is a natural science that deals with the composition of matter and the changes it undergoes. Which of the following are examples of matter? ◗ concrete ◗ acetone (propanone) vapour ◗ heat ◗ sound ◗ air ◗ light Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 7 Laboratory Techniques for Environmental Technicians Matter is anything that has mass and occupies space. Matter can exist as solid, liquid, gas and plasma. Some Classification Schemes Solids/ Liquids/ Gas Matter can exist in three states, solid, liquid or gas (and recently a fourth state, plasma has been recognised). Gas Liquid Solid Describe how the particles are arranged in each of the three states identified in the diagram above Type you answer here Leave blank for assessor feedback Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 8 Laboratory Techniques for Environmental Technicians Characteristics of the states of matter Gas Liquid Solid Particle position widely spaced, less closely spaced, closely move freely some collisions vibrate position Shape varies varies-takes shape definite of container Volume varies definite definite Compress- Easily compressed slightly Negligible rapid slow negligible in packed, fixed ibility Diffusion In which state of matter do the following exist at room temperature and pressure? ◗ diamond ◗ oxygen ◗ cooking oil ◗ mercury ◗ carbon dioxide ◗ sugar ◗ Concrete ◗ Oxygen ◗ Air ◗ Nitrogen Type you answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 9 Laboratory Techniques for Environmental Technicians Pure/ Impure A pure substance is either an element or a compound. A pure substance is identified by its constant physical properties. Elements are substances composed of only one type of atom. They have fixed physical properties. The known elements have been arranged into a table known as the Periodic Table. Depending on the element the atoms could be arranged in one of the following; ◗ Monatomic, composed of only one atom for example helium ◗ Diatomic, composed of two atoms of the same element for example oxygen ◗ Triatomic, composed of three atoms of the same element for example ozone ◗ Polyatomic, composed of many atoms of the same element for example diamond, graphite Where elements can exist in more than one form they are referred to as allotropes of an element for example: ◗ Carbon in the form of graphite, diamond, bucky balls ◗ Oxygen in the form of oxygen gas and ozone gas ◗ Phosphorus in red and white forms List any elements that you are familiar with. Use a periodic table to find the position on the table and also the symbol. (A periodic table is available in the Appendix file) Element Symbol Oxygen O Answer Answer Answer Answer Answer Answer Element Symbol Answer Answer Answer Answer Answer Answer Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 10 Laboratory Techniques for Environmental Technicians Use a periodic table to fill in the comical ‘Gold Dust Kid’ activity An Elemental Tale: The Gold Dust Kid The Kid mounted his trusty steed, old (B) ……………. His shooting (Fe)…………… strapped to his side, he headed out for the bright (Ne)…………… lights of Sabattus, aiming to rob the Litchfield stage. There was sure to be a load of precious (U)……………… aboard, and probably (K)……………………too. Inhaling a deep breath of (O) ………………. he coughed on the (S)……………….. from the nearby hills. Since the (Hg) …..…………….was climbing, he quenched his thirst with some H2O, tasting the (Cl) ……………… from (Ca) ……………….. deposits built up over the years of riding the (Zn) ……………. trail. Overhead a (He) …………..-filled balloon floated in the breeze; the sun beat down like burning (P)…………… Soon he spotted the stage, guarded only by a sheriff with a (Sn)……………badge. “Halt”, he yelled, “or I’ll fill you full of (Pb)……………. The sheriff drew his gun, but alas, was too slow. The Kid’s gun blazing like flaming (Mg)…………..……… did the (Cu)……………….. in. Anyone who drew on the Kid should know his life wasn’t worth a plugged (Ni)…………….. A (Pt) ………………… blonde riding beside the (Al) …………………..-framed coach rode for her life when the Kid pulled out some (N) ………………… compounds, preparing to blow the safe to atoms. Suddenly a shout rang out, “Hi Ho (Ag)…………….. and a masked man on a white horse raced across the (Si) ……………….. sands like (Na) ……………….. skittering on water. A (H) ………………… bomb would not have stopped the lawman; the Kid had met his doom. The rest of his life was to be spent behind (Co) ………………. steel bars, a warning to all who flirt with danger. Your first detention may be the initial step in a (C) ……………..copy of the saga of the (Au) ………………. Dust Kid. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 11 Laboratory Techniques for Environmental Technicians Practical work Complete the following table by observing samples of elements that you may have access to at home What criteria did you use to decide that an element was a metal or a non-metal? Type you answer here Leave blank for assessor feedback Compounds are substances composed of two or more atoms chemically combined in a fixed ratio. Elements are always present in the same ratio in a given compound. The properties of a compound are usually quite different from those of the elements from which it is composed. Chemical methods are required to separate compounds into their constituent elements. List any compounds that you are familiar with eg water Type you answer here Leave blank for assessor feedback A mixture is a physical combination of two or more substances. A mixture has a variable composition and may be identified as heterogeneous or homogeneous. Mixtures can be separated into pure substances using methods based on differences in their physical properties. Examples a magnet can be used to separate iron from other solids……magnetic properties solids and liquids can be separated by decanting, filtering and centrifuging……. differences in density or particle size two immiscible liquids can be separated using a separating funnel List some mixtures that you are familiar with. Type you answer here Leave blank for assessor feedback Homogeneous mixtures (solutions) have uniform properties throughout. Solutions may be gases, liquids or solids. Like all other mixtures solutions have variable composition. Heterogeneous mixtures are those that do not have uniform properties throughout. Mixtures can be easily separated: Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 12 Laboratory Techniques for Environmental Technicians Laboratory techniques for separating mixtures METHOD MIXTURE TYPE DIAGRAM (property differences) Decanting Used to undissolved separate sediment from a liquid (density) or immiscible liquids. Separating funnel used to separate immiscible liquids (density) Filtration Gravity and techniques used to separate an Vacuum undissolved solid from a liquid. (particle size) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 13 Laboratory Techniques for Environmental Technicians METHOD MIXTURE TYPE DIAGRAM (property differences) used to separate undissolved substances (some liquid) of differing densities Centrifuge (density) Evaporation Crystallisation / used to separate dissolved solid from solvent (liquid) Distillation a a used to separate mixtures of liquids (boiling point) Chromatography used to separate miscible liquids (partition – differences in attraction of sample matrix for mobile phase Vs stationary phase) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 14 Laboratory Techniques for Environmental Technicians METHOD MIXTURE TYPE DIAGRAM (property differences) Which laboratory method would you use to separate the following mixtures? ◗ charcoal and salt ◗ water and oil ◗ alcohol and water ◗ sugar from a sugar solution ◗ water from muddy river water Type you answer here Leave blank for assessor feedback Classify each of the following as element, compound or mixture ◗ soil ◗ silver ◗ milk ◗ table sugar ◗ sulfur ◗ river water ◗ grape juice ◗ nitrogen ◗ fog Type you answer here Leave blank for assessor feedback Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 15 Laboratory Techniques for Environmental Technicians A change in the properties of a substance without a change in composition is a physical change. Physical changes include melting and boiling eg water to ice and steam Changes of State We can show changes of state in the following way: If there is a change in the composition of a substance, however, a chemical change is indicated. In a chemical change reactants are converted to products eg the breakdown of water into its elements hydrogen and oxygen. In any physical or chemical change, both mass and energy are conserved. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 16 Laboratory Techniques for Environmental Technicians Practical: Physical versus chemical change Purpose: To distinguish a physical from a chemical change based on observation. Task: (Teacher demonstration) ◗ You will be required to record observations for each set up and identify each as either a physical or chemical change. ◗ Summarise the process(es) taking place at each station, expressing each in terms of starting and finishing substances. Expressing physical states as subscripts next to each substance will assist in this regard. ◗ Describe the demonstrations of the reactions. State whether a chemical or physical change takes place. o Zinc and acid o Copper and acid o Sodium and water o Sulfur, air and a flame o Magnesium, air and a flame o Iodine and heat o Boiling water o Electrolysis of water o Hydrogen, air and flame Question What observations could you use to assist in determining if a chemical or physical change is occurring? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 17 Laboratory Techniques for Environmental Technicians Atomic Structure Plato, Aristotle 500 BC All matter composed of four elements, earth, wind, air and fire Democritus 400BC Matter not infinitely divisible, but was made of extremely small hard particles called atoms which were indivisible Cavendish 1700’s Made water from hydrogen and oxygen disproving previous theory Lavoisier 1700’s Law of conservation of mass: matter cannot be created or destroyed Proust 1799 Law of constant composition: a compound always contains the same elements in the same ratios by mass Dalton 1808 1. Matter is composed of tiny indivisible particles called atoms 2. All atoms of the one element are the same but different to atoms of other elements 3. Chemical reactions consist of combining , separating or rearranging atoms in simple whole number ratios Thomson 1904 Discovery of electrons as particles within atoms. Proposed “plum pudding” model, where electrons were embedded in a sphere of positive charge Rutherford 1911> Famous gold leaf experiment: 1. Matter is mostly empty space 2. The atoms consists of a small dense +ve nucleus containing most of the mass of the atom 3. The nucleus is surrounded by electrons making it electrically neutral Chadwick 1932 Discovered the existence of neutrons Bohr 1913> Proposed models of atom which contained allowable orbits or shells for electrons surrounding the nucleus. Many other contributions have been made by famous scientists such as Plank, Schrodinger, Oppenheimer etc. The model of the atom is being constantly refined. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 18 Laboratory Techniques for Environmental Technicians Bohr model of the atom Proposed by Neils Bohr; ◗ central massive body, nucleus, (like the sun in our solar system) ◗ The nucleus has a positive charge ◗ very small electrons circling, (like the planets) Terms related to atomic structure Term Definition Atomic number (Z) the number of protons in an atom. Identifies the element Atomic weight average mass of naturally occurring isotopes of an element Electrons negatively charged particles orbiting the nucleus Ions a charged atom Isotopes atoms of the same element with differing numbers of neutrons Mass number (A) the number of protons plus the number of neutrons in an atom Neutrons particles found in the nucleus but with no charge Protons positively charged particles found in the nucleus Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 19 Laboratory Techniques for Environmental Technicians An element may be represented in the Periodic Table using a shorthand notation: ZXA Where: X = element symbol Z = atomic number, number of protons A = mass number Neutron number = mass number - atomic number = A-Z Particle Relative charge Relative mass Location proton +1 1 au Inside the nucleus neutron 0 1 au Inside the nucleus Electron -1 0 Orbiting the nucleus Use your periodic table to complete the following table, remembering the following information Atomic number = identity of atom = number of protons = no of electrons(for an uncharged atom) Mass number = no of protons + number of neutrons Neutrons = Mass number – number of protons Element Atomic (Z) hydrogen 1 1 Carbon 6 Text Text Text Text Text 26 Text Text Text Text Text Text No. Mass No. (A) Protons (p) Electrons (é) Neutrons (n) 1 1 0 13 6 6 7 Text Text 11 12 22 11 Text Text Text Text Text Text 210 197 Text Text Text 36 Text 79 29 48 128 Text Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 20 Laboratory Techniques for Environmental Technicians How are the electrons arranged around the nucleus? For this study we will use only the simplified version and only look at the first 20 elements. Each shell (or orbit) about the nucleus has a specific number of electrons that can be held. In electron, in an unexcited atom, ground state) can only be placed in a shell in a certain order. A shell must be filled before a new shell can commence. The pattern for filling the shells follows the following pattern: ◗ Shell 1 can contain a maximum of 2 electrons ◗ Shell 2 can contain a maximum of 8 electrons ◗ Shell 3 can contain a maximum of 8 electrons ◗ Shell 4 can contain a maximum of 18 electrons Electron configuration of the first 20 elements on the Periodic Table Atomic No Element Protons Electrons 1 Hydrogen 1 1 (found shell) 2 Helium 2 Lithium 3 in 1st in 2nd e 2 (found shell 3 Shell diagram e 3 e Text (2 electrons in 1st shell + 1 electron in 2nd shell) 4 Text Text Text Text 5 Text Text Text Text 6 Text Text Text Text 7 Text Text Text Text 8 Text Text Text Text Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 21 Laboratory Techniques for Environmental Technicians 9 Text Text Text Text Text 10 10 Text 10 11 (2 electrons in 1st shell + 8 electrons in 2nd shell, which is now full) Sodium 11 11 Text (2 electrons in 1st shell + 8 electrons in 2nd shell + 1 electron in 3rd shell) 12 Text Text Text Text 13 Text Text Text Text 14 Text Text Text Text 15 Text Text Text Text Text Text Text Text 17 Text Text Text Text 18 Text Text Text Text 19 Text 19 19 Text 16 (2 electrons in 1st shell + 8 electrons in 2nd shell + 8 electrons in 3rd shell + 1 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 22 Laboratory Techniques for Environmental Technicians electron 4thshell) 20 Text Text in Text Text Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 23 Laboratory Techniques for Environmental Technicians How can atoms become charged? In simple terms the only part of an atom that is able to move is the electron. Electrons can be added or removed from an atom. If this occurs then the atom is no longer neutral and a charged particle, called an ion is formed. If electrons are lost the atom becomes positively charged and is termed a cation If electrons are gained the atom becomes negatively charged and is termed an anion. For example. Sodium atom: ◗ has 11 protons and a nuclear charge = +11 ◗ has 11 electrons and an electron charge = -11 ◗ overall net charge = 0 Sodium loses an electron easily forming a sodium cation ◗ 11 protons and a nuclear charge = +11 ◗ Now has 10 electrons and an electron charge = -10 ◗ Overall net charge = +1 Oxygen atom: ◗ Has 8 protons and a nuclear charge = +8 ◗ Has 8 electrons and an electron charge = -8 ◗ Overall net charge = 0 Oxygen can gain two electrons easily forming a oxygen anion ◗ Has 8 protons and a nuclear charge = +8 ◗ If it gains 2 electrons the electron charge = -10 ◗ Overall net charge = -2 Explain how the following can occur Explanation Text Chlorine has -1 charge Text Magnesium has +2 charge Text Phosphorus has -3 charge Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 24 Laboratory Techniques for Environmental Technicians Text Aluminium has + 3 charge Text Sulfur has -2 charge Text Caesium has +1 charge Is there a pattern you can see in the relative charges of the metals and non-metals? Type you answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 25 Laboratory Techniques for Environmental Technicians Terms and Definitions (From Chemistry for Technicians) Terms A proton B electron C neutron D atomic number E isotope F mass number G atomic weight H ion I anion J cation K monatomic ion L nucleus M valence shell Definitions 1 The number of protons in an atom Term 2 A negatively charged ion Type your answer here 3 A positively charged subatomic particle Type your answer here 4 Atoms with the same number of protons, Type your answer here but different numbers of neutrons 5 A charge atom or molecule Type your answer here 6 An ion containing a single atom Type your answer here 7 The centre of the atom, which contains Type your answer here most of the mass 8 A negatively charged subatomic particle Type your answer here 9 A positively charged ion Type your answer here 10 The combined total of protons and neutron Type your answer here 11 The outermost shell containing electrons Type your answer here 12 A subatomic particle with no charge Type your answer here 13 The average mass of naturally occurring Type your answer here atoms of an element Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 26 Laboratory Techniques for Environmental Technicians The Periodic Table & Atomic Structure The periodic table is an arrangement of the known elements. The modern periodic table has the elements arranged in order of increasing atomic number…….the early versions had the elements arranged according to atomic mass. Can you find examples where this would be different to the modern version? The periodic table briefly Independently arrived at by Mendeleev and Meyer in the mid-19th century. Mendeleev predicted the properties of undiscovered elements and left spaces in his periodic table. Consists of a series of horizontal rows, periods and vertical columns, groups: Group 1: Alkali metals Group 2: Alkaline earth metals Group 7: Halogens Group 8: Inert gases provides a summary of atomic weights and numbers, symbols and names for the elements understanding of properties of related elements Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 27 Laboratory Techniques for Environmental Technicians Chemical Formulae A chemical symbol represents one atom of an element. A chemical formula represents one molecule or one formula unit of an element or compound The formula shows: ◗ Each type of atom present ◗ The number of each atom present in the molecule or formula unit ◗ Coefficients, superscripts and subscripts where necessary A molecule is a group of atoms which are bonded together…the atoms may be the same or different. Examples: Elements Compounds H2 = hydrogen H2O P4 = phosphorus C6H12O6 = glucose O2 = oxygen NH3 = water = ammonia A formula unit represents the simplest ratio of atoms in a substance, which may be a giant lattice Examples: Substance Type Formula Diamond Element C Sodium chloride Compound (ionic) NaCl Silica Compound (covalent) SiO2 Ions (charged particles) use superscripts to show the electrical charge. Example Mg2+ = a magnesium ion having a charge of +2 CO32- = one carbon atom and three oxygen atoms forming an ion, carbonate ion, with a charge of –2 For each of the following describe the information each formula is providing about the elements and how many of them there are. CH4 = 1 carbon atom and 4 hydrogen atoms Sn3(PO4)2 = CuSO4.5H2O = Cl- = OH- = Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 28 Laboratory Techniques for Environmental Technicians The elements and their symbols (arranged alphabetically by symbol) Ac Ag Al Am Ar As At Au B Ba Be Bi Bk Br C Ca Cd Ce Cf Cl Cm Co Cr Cs Cu Dy Er Es Eu F Fe Fm Fm Ga Gd Ge H He Hf Hg Ho actinium silver aluminium americium argon arsenic astatine gold boron barium beryllium bismuth berkelium bromine carbon calcium cadmium cerium californium chlorine curium cobalt chromium caesium copper dysprosiu m erbium einsteiniu m europium fluorine iron fermium francium gallium gadolinium germanium hydrogen helium hafnium mercury holmium I In Ir K Kr La Li Lr Lu Md Mg Mn Mo N Na Nb Nd Ne Ni No Np O Os P Pa Pb iodine indium iridium potassium krypton lanthanum lithium lawrencium lutetium mendelevium magnesium manganese molybdenum nitrogen sodium niobium neodymium neon nickel nobelium neptunium oxygen osmium phosphorus proactinium lead Pd P m Po Pr Pt Pu Ra Rb Re Rh Rn Ru S Sb Sc palladium promethium Se Si Sm Sn Sr Ta Tb Tc Te Th Ti Tl U Unq Unp Unh V W Xe Y Yb Zn Zr selenium silicon samarium tin strontium tantalum terbium technetium tellurium thorium titanium thallium uranium 104 in dispute 105 in dispute 106 in dispute vanadium tungsten xenon yttrium Ytterbium Zinc Zirconium polonium praseodymium platinum plutonium radium rubidium rhenium rhodium radon ruthenium sulfur antimony scandium Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 29 Laboratory Techniques for Environmental Technicians Systematic naming of inorganic compounds Inorganic compounds are generally of two types: ◗ Ionic (generally composed of a metal ion and a non-metallic ion eg sodium chloride, NaCl) ◗ Molecular (generally composed only of non-metals eg nitrogen dioxide, NO2) To manipulate formulae, it is necessary to: ◗ learn the symbols for the common elements (or be able to obtain them from the periodic table) ◗ learn the common ions and their charges ◗ identify types of formulae. learn to name compounds correctly. Common ions and their charges +1 +2 +3 +4 -1 -2 -3 ammonium NH4+ barium Ba2+ aluminium Al3+ phosphate PO43- calcium Ca2+ Copper (II) Cu2+ iron (II) Fe2+ lead (II) Pb2+ magnesium Mg2+ iron (III) Fe3+ acetate (ethanonate) CH3COO bromide Br chlorate ClO3 chloride Cl fluoride Fhydrogen carbonate HCO3hydrogen sulfate HSO4 hydroxide OH iodide Initrate NO3 nitrite NO2 Permanganate MnO4- carbonate CO32- potassium K+ silver Ag+ sodium Na+ Hydrogen H+ Lead (IV) Pb4+ tin (IV) Sn4+ chromate CrO42dichromate Cr2O72oxide O2peroxide O22sulfate SO42- Phosphide P3Nitride N3- mercury(II) Hg2+ nickel Ni2+ tin (II) Sn2+ sulfite SO32sulfide S2- Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 30 Laboratory Techniques for Environmental Technicians Types of compounds The simplest compounds contain only two elements and are called binary compounds. a) Metal-non-metal binary compounds (ionic compounds) The metal is written as the first name and the non-metal ending “ide” forms the last name eg Na2S sodium sulfide MgO magnesium oxide If more than one compound of the metal exists, their oxidation numbers are used eg FeCl2 iron (II) chloride and FeCl 3 iron (III) chloride Name the following metal-non-metal compounds (check for a metal then it is as simple as writing the metal name followed by the non-metal ending. ZnO Type your answer here K2O Type your answer here Al2O3 Type your answer here Ca3(PO4)2 Type your answer here Complete the table below by writing in the name of the metal and the non-metal ending then combining them together Ions Li+ NH4+ Mg2+ Lithium Br - Al3+ Na+ Aluminium Text Text Text Text Bromide Lithium bromide SO42- Text Text Text Text Text OH - Text Text Text Aluminium hydroxide Text F- Text Text Text Text Text Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 31 Laboratory Techniques for Environmental Technicians O2- Text Text Text Text Text NO3 - Text Text Text Text Text PO43- Text Text Text Text Text Cl - Text Text Text Text Text S2- Text Text Text Text Text I- Text Text Text Text Text CO32- Text Text Text Text Text HCO3 - Text Text Text Text Text CH3COO- Text Ammonium acetate Text Text Text (ammonium ethanoate) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 32 Laboratory Techniques for Environmental Technicians Chemical formulae of ionic compounds The valency of an atom or polyatomic ion can be viewed as a bonding position which must be filled to form a stable compound. Recap: in order to find the formula of a compound you must recognise the parts or constituents from the name. Since the name is in two parts this recognition is easy as long as you realise that the ending of the name of the second atom is often changed to -ide. Oxygen becomes oxide; sulfur becomes sulfide; chlorine becomes chloride etc. Na a Na Cl Cl will join the ratio 1:1 Sodium’s valency is 1+ Formula is Na1Cl1 or Chloride’s valency is 1- NaCl (1 is automatically assumed) 2. Aluminium nitrate is a compound made from aluminium and nitrate polyatomic ion Al NO3 NO3 Al will join in the ratio 1:3 NO3 Aluminium has a valency of 3+ NO3 Nitrate has a valency of 1Formula will be Al(NO3)3 3. Lead IV oxide is a compound made from lead (valency 4) and oxygen. Pb O will join in the ratio 1:2 Pb O Lead has a valency of 4+ Oxygen has a valency of 2The formula will be PbO2 O Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 33 Laboratory Techniques for Environmental Technicians Cross Multiply Method Step 1. Write the symbols which represent each part of the name. Step 2. Write the valencies as a superscript to each part. Step 3. Cancel by dividing the valencies by any common factor Step 4. Cross over the numbers to form subscripts, using brackets where necessary. Examples: 1+ 1. Na Sodium Chloride 1- Cl 3+ 2. Aluminium Nitrate Al 1- NO3 4+ 3. Lead (IV) Oxide Pb 2 1 Formula is Na1Cl1 or NaCl Formula is Al1(NO3)3 or Al(NO3)3 2- O Formula is Pb1O2 or PbO2 4 2 Write chemical formulae for the following compounds. Compound Formula Compound Formula calcium chloride Text potassium sulfite Text magnesium oxide Text nickel carbonate Text lead (IV) sulfate Text silver phosphate Text Text potassium nitrate Text Text sodium carbonate Text Text ammonium chloride Text barium phosphide zinc sulfate iron (III) hydroxide Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 34 Laboratory Techniques for Environmental Technicians b) Two non-metal binary compounds (molecular compounds) The first name is the more electropositive element and the other non-metal is given the -ide ending for the surname. The prefixes: ◗ mon =1 ◗ di = 2 ◗ tri = 3 ◗ tetra =4 ◗ penta =5 ◗ hexa =6 are used to show the proportions of each element. eg N2O3 dinitrogen trioxide SO2 sulfur dioxide The prefix mono is used only when there is more than one compound of the two elements eg CO carbon monoxide CO2 carbon dioxide Write the formula for the following non-metal binary compounds Compound Formula Compound Formula nitrogen triiodide Text silicon dioxide Text silicon tetrafluoride Text water Text Diphosphorus pentoxide Text ammonia Text c) Common names are given to some hydrides H2O water NH3 ammonia CH4 methane Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 35 Laboratory Techniques for Environmental Technicians d) Composite ion (radical) compounds The positive ion is written as the first name and the negative ions the surname. eg NH4Br ammonium bromide NaOH sodium hydroxide (NH4)2SO4 ammonium sulfate e) Acids i) Halogen acids eg HCl(g) HCl(aq) hydrogen chloride hydrochloric acid ii) Oxygen containing acids If only one acid exists, the ending -ic is used eg hydrofluoric acid HF carbonic acid H2CO3 If two acids exist the lowest oxidation state ending is -ous and the higher state ending is -ic. eg sulfurous acid H2SO3 sulfuric acid H2SO4 The formula for the some common acids is provided below. (In some cases the common name has also been given). Name Common name Formula Hydrochloric acid Muriatic acid HCl Sulfuric acid Oil of vitriol H2SO4 Phosphoric acid Text H3PO4 Nitric acid Text HNO3 Sulfurous acid Text Text Ethanoic acid Acetic acid Text Boric acid Text Text Carbonic acid Text Text Phosphoric acid Text Text Name the following compounds (Remember to identify the type of compound first eg is there a metal …… if yes then just name the metal and the ending!) Formula Name Formula Name Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 36 Laboratory Techniques for Environmental Technicians Ni(NO3)2 Text SO3 Text PCl3 Text FeSO4 Text P2O5 Text Fe2(SO4)3 Text Fe2O3 Text PbO Text CrCl3 Text PbO2 Text IF7 Text Ca3N2 Text KHSO4 Text NO2 Text Li3PO4 Text N2O4 Text NaNO2 Text Ca(OH)2 Text LiH Text Na2CO3 Text S2Cl2 Text Text Complete the following table Name Formula Name Formula copper (II) oxide Text Ammonia Text oxygen difluoride Text potassium permanganate Text copper (II) sulfate Text hydrogen chloride Text barium hydroxide Text dihydrogen sulfide Text copper (I) bromide Text Silicon dioxide Text aluminium hydroxide Text phosphorus pentachloride Text dinitrogen tetroxide Text chromium (III) fluoride Text Silver bromide Text Text Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 37 Laboratory Techniques for Environmental Technicians Organic chemicals Organic chemistry involves the study of carbon compounds which contain hydrogen and sometimes oxygen, chlorine, nitrogen etc. Many of out useful organic chemicals are obtained from coal and oil or synthesised from chemicals obtained from them or plants and animals. Coal benzene, toluene, naphthalene, creosote Petroleum methane, propane, octane, petrols, oils, kerosene, paraffin, bitumen Plants sugar, starch, edible oils, waxes, gelatin, dyes, drugs, natural fibres These raw materials are either used directly or converted to others eg plastics, pharmaceuticals, dyes, pigments, cosmetics, insecticides, explosives, refrigerants, paints etc Carbon forms so many compounds because of its ability to form strong bonds to itself and other elements. IT ALWAYS HAS 4 BONDS (places where other elements can join) The other elements carbon bonds with include; ◗ Hydrogen 1 bond ◗ Oxygen 2 bonds ◗ Nitrogen 3 bonds ◗ Chlorine 1 bond ◗ Bromine 1 bond ◗ Iodine 1 bond Carbon can also form multiple bonds with itself and oxygen and nitrogen Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 38 Laboratory Techniques for Environmental Technicians Carbon compounds are named according to the family they belong to according to their structure. Family Structure contains Alkanes Single bonds between C Methane atoms Butane Alkenes Double bond carbons between Ethene Triple bond carbons between Ethyne Alkynes Alkanols (alcohols) Example Butene Contain –OH Butyne Methanol Butanol Alkanals (aldehydes) Contain C=O on the end Methanal Butanal Alkanones (ketones) C=O within the structure Propanone Butanone Alkanoic acids Methanoic acid Butanoic acid Esters Methyl butanoate Butyl methanoate Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 39 Laboratory Techniques for Environmental Technicians Writing Chemical Equations - Chemical Shorthand A chemical equation is a shorthand method of communicating information about reactants and products, quantities involved and sometimes how much energy is needed or released. It accounts for all the atoms involved in the re-arrangement of bonds. During a chemical reaction, bonds are broken in the reactants and new bonds are formed making the products. The atoms are re-arranged during a reaction, but the numbers of the different types of atoms remain constant. The law of Conservation of Matter (or mass) states that during a chemical reaction the total mass of reactants is the same as the total mass of products formed. During a chemical reaction, matter is neither created nor destroyed, it is only changed from one form into another. Law of Conservation of Mass (Matter) Note: The total number of atoms of each element is unchanged! Example 1: When a spark ignites a mixture of hydrogen gas and oxygen gas, an explosion occurs - a large amount of energy is released. The product formed is water. Chemical reactions which release energy are called exothermic. Word Equation: Hydrogen(g) + Oxygen(g) ⇌ Water(l) + energy Symbol Equation: H2(g) + O2(g) ⇌ H2O(l) + energy (unbalanced) The correct chemical formula is written under each reactant and product. The extra subscripts indicate the state of that substance. The equation above is said to be unbalanced because the numbers of various atoms represented on the reactants’ side (left of the arrow) is not the same as those on the products’ side (right of the arrow). This would mean that mass is not conserved! Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 40 Laboratory Techniques for Environmental Technicians Reactants ( H2 + O2 ) Products ( H2O ) H=2 H=2 O=2 O=1 The numbers of oxygen atoms are not balanced! We must adjust the numbers of molecules of each type present to reach a balance! This involves placing numbers where required in front of each formula. The number will multiply each atom in the formula. This was referred to previously as a coefficient number. A “2” in front of the H2O product will provide the required 2 oxygen atoms on the right. H2(g) + O2(g) ⇌ 2H2O(l) + energy Reactants ( H2 + O2 ) Products ( 2 H2O ) H=2 H=4 O=2 O=2 Whilst oxygen has now been balanced, Hydrogen is now unbalanced! In order to balance the hydrogen atoms it is necessary to place a “2” in front of the H2 on the left. This provides 4 hydrogen atoms on the left also. Balanced Equation: 2H2(g) + O2(g) ⇌ 2H2O(l) + energy Reactants ( 2 H2 + O2 ) Products ( 2 H2O ) H=4 H=4 O=2 O=2 Example 2 When an emergency flare lights up, magnesium (or aluminium) metal combines with oxygen gas releasing a bright light. This is also an exothermic reaction. The substance formed is a metal oxide. Word equation: Magnesium(s) + Oxygen(g) ⇌ Magnesium oxide(s) + energy Symbols: Mg(s) + O2(g) ⇌ MgO(s) + energy Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 41 Laboratory Techniques for Environmental Technicians Reactants ( Mg + O2 ) Products ( MgO ) Mg = 1 Mg = 1 O=2 O=1 The numbers of oxygen atoms are not balanced! Balance the oxygen atoms by placing a coefficient “2” in front of MgO. This will also produce 2 magnesium atoms. Mg(s) + O2(g) ⇌ 2MgO(s) + energy Reactants ( Mg + O2 ) Products ( 2 MgO ) Mg = 1 Mg = 2 O=2 O=2 Then balance the magnesium atoms by placing a coefficient “2” in front of Mg. Balanced equation: 2 Mg(s) + O2(g) ⇌ 2MgO(s) + energy Reactants ( 2 Mg + O2 ) Products ( 2 MgO ) Mg = 2 Mg = 2 O=2 O=2 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 42 Laboratory Techniques for Environmental Technicians Written Exercises 1. Balance the following chemical equations. (a) CH4(g) + O2(g) ⇌ CO2(g) + H2O(l) (b) Na(s) + H2O(l ) ⇌ NaOH(aq) + H2(g) (c) Zn(s) + HCl(aq ) ⇌ ZnCl2(aq) + H2(g) (d) P + O2 ⇌ P2O5 (e) NH3 + H2SO4 ⇌ (NH4)2SO4 (f) CuO + HCl ⇌ CuCl2 + H2O (g) H2O2 ⇌ H2O + O2 (h) H2CO3 ⇌ H2O + CO2 (i) Fe + O2 ⇌ Fe2O3 (j) C8H18 + O2 ⇌ CO2 + H2O The Mole Concept We need to “scale up” our quantities from the molecular level to an amount which we can see and measure in the laboratory. The quantity of particles chosen is called the mole which is approximately 6.02 x 1023 particles of any pure substance (ie; element or compound). Scientific notation is expressed in this number as this is a gigantic quantity! The Periodic Table measures the mass of each element in atomic units (a.u.) One mole of any substance contains 6.02 x 1023 particles and has a mass measured in grams equal to its formula weight (a.u.) One mole of water, H2O contains 6.02 x 1023 molecules. The mass of 1 mole of water is equal to its formula weight (FW), expressed in grams; FW (Hydrogen) = 1.008 a.m.u. FW (Oxygen) = 16.00 a.m.u. ∴ (2 x 1.008) + 16.00 = 18.016 g. Likewise, one mole of hydrogen (gas) contains 6.02 x 1023 molecules of hydrogen and has a mass of 2 x 1.008 = 2.016 g. And one mole of oxygen (gas) contains 6.02 x 1023 molecules of oxygen and has a mass of 2 x 16.00 = 32.00 g. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 43 Laboratory Techniques for Environmental Technicians We could, if we wanted, determine the ratio, by mass of the substances involved in the decomposition of water. ⇌ 2H2O(l) 2 molecules 2H2(g) + 2 molecules of water O2(g) 1 molecule of hydrogen of oxygen 2 moles 2 moles 1 mole of water of hydrogen of oxygen 2 x 18.016 2 x 2.016 1 x 32.00 = 36.032 g = 4.032 g = 32.00 g Therefore; If we started with 1 mole (ie 36.032 g) of water, we would obtain 4.032 g of hydrogen and 32.00 g of oxygen. What mass of hydrogen and oxygen would we obtain if we started with 1.00 g of water? H2O (l) H2 (g) O2 (g) 36.032 4.032 32.00 36.032 36.032 36.032 Therefore 1.00 g ⇌ 0.11 g + 0.89 g If we started with 1.00 g of water we would obtain 0.11 g of hydrogen and 0.89 g of oxygen. Notice that the balancing numbers do not give us masses directly, that is we cannot say that 2 g of water will give 2 g of hydrogen and 1 g of oxygen! When we use the mole concept we are taking into account that the various atoms have different masses, and the balancing numbers give us the right ratios of atoms involved. It makes life much easier when interpreting number coefficients of a balanced chemical equation as mole quantities, because that’s exactly what they are ! Take special note that the total mass of reactants equals the total mass of products. No atoms are lost or gained, as understood from our work on writing and balancing chemical equations. Atoms are just re-arranged in a chemical reaction. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 44 Laboratory Techniques for Environmental Technicians We could continue our calculations to account for any mass of water, or we could calculate the quantity of water required to produce a particular amount of hydrogen or oxygen. The mole ratios of reactants to products are determined from the balanced chemical equation and a knowledge of the mole concept. We can determine the number of moles present in a sample of a substance by using one or more of the following formulae: No of moles = No of particles 6.02 x 1023 This mole equation will not be immediately useful as it deals with a known number of chemical particles, which in reality is mostly unrealistic. You may, however be asked to calculate a mole quantity given such data. No of moles Mole Equation 1. = Mass (g) Formula Weight This equation is useful in the real world, as mass and Formula Weight are measurable quantities in the real world. Shorthand: n = m FW Written Exercises: Mole calculations 1. Calculate the Formula Weight, (FW) for each of the following substances for which the formula is provided. (a) Sodium Chloride, NaCl Type your answer here (b) Ammonium Sulfate, (NH4)2SO4 Type your answer here (c) Glucose, C6H12O6 Type your answer here (d) Potassium Hydrogen Phthalate, KC8H5O4 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 45 Laboratory Techniques for Environmental Technicians Type your answer here 2. Calculate the number of moles of each substance (a) 58 g of NaCl Type your answer here (b) 13.2 g of (NH4)2SO4 Type your answer here (c) 90 g of C6H12O6 Type your answer here (d) 5.0 g of KC8H5O4 Type your answer here 3. What mass of each substance is present? (a) 2.5 moles of NaCl Type your answer here (b) 0.5 moles of (NH4)2SO4 Type your answer here (c) 4 moles of C6H12O6 Type your answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 46 Laboratory Techniques for Environmental Technicians Solutions When a substance dissolves in a liquid we say a solution has been formed . The substance can be a solid, liquid or gas and is called the solute. The liquid it dissolves in is called the solvent. The strength or concentration of a solution can be described by the number of moles of solute in 1 litre of solution (other units may be % or g per litre). An aqueous solution is the term applied for a solution made of a solute with water as the solvent. A 1 molar solution (1M) has 1 mole of solute dissolved in 1 litre of solution Complete the following: A 0.5 molar sucrose solution (0.5M) has 0.5 moles of sucrose in 1 litre of solution. A 2.5 M salt solution hasClick here to enter text.moles of salt in 1 litre of solution A 0.001 M NaOH solution hasClick here to enter text. moles of Click here to enter text.in 1 litre of solution A 0.2 M KI solution has Click here to enter text.in 1 litre of solution. No. of moles of solute = (Concentration of solution) X (Volume of solution) (M) X (L) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 47 Laboratory Techniques for Environmental Technicians Calculating the molarity of a solution The number of moles (n) of solute per litre (L) of solvent is referred to as the MOLARITY of a solution. Molarity can be written as mol/L, but it is more commonly written as M. Hence when you are given a bottle marked ‘0.1M NaOH’ this means that the solution consists of 0.1 mole of sodium hydroxide per litre of solvent. This solution is referred to as being a 0.1 Molar solution. [ ] represents concentration in moles/L Molarity = moles (moles/L) litres Examples 1.What is the molarity of a solution containing 0.25 moles of NaOH in 1.2 L of water. [ NaOH] = 0.25 moles 1.2 L = 0.208 mol/L 2. How many grams of NaOH are required to make 2 litres of a 0.15 M solution? a. Firstly calculate how many moles of NaOH are required to make up the solution. Rearrange the equation to make ‘moles’ the subject of the formula moles (n) = Molarity (M) x Litres (L) moles = 0.15 x 2 moles = 0.3 b. How many grams of NaOH are there in 0.3 mole of the substance? moles = mass (g) FWt Rearrange equation to make mass the subject. mass = moles x FWt Calculate the formula weight of NaOH = 39.998 Mass = 0.3 x 39.998 Mass = 11.999g NaOH Therefore, 11.999g NaOH are required to prepare 2 L of 0.15 M NaOH Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 48 Laboratory Techniques for Environmental Technicians Exercises 1. Determine the molarity of a solution in which 4.67 moles of Li2SO3 are dissolved to make 2 L of solution Type your answer here 2. Determine the molarity of a solution in which 4.783 g of Na2CO3 is dissolved to make 10 L of solution. Type your answer here 3. Determine the number of moles of solute needed to prepare 16.00 mL of a 0.415 M Pb(NO3)2 solution. Type your answer here 4. Determine the mass of solute required to prepare 0.500L of a 1.00 M KCl solution. Type your answer here 5. Determine the final volume of the solution needed when 8.07g of (NH4)2CO3 is dissolved to make a 0.250 M solution. Type your answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 49 Laboratory Techniques for Environmental Technicians Dilution of solutions Many laboratories will keep a range of commonly used solutions, often at a fairly high Molarity. One of your tasks may be to prepare a solution of lower Molarity from one of these ‘stock’ solutions. You will be diluting the stock solution. To dilute a solution means to add more solvent without the addition of more solute. There is an equation which relates the Molarity and Volume of the stock solution to the Molarity and Volume of the solution you wish to prepare. C1V1 = C2V2 where C = concentration, which is expressed as molarity or moles/L V = volume which is expressed in litres C1 refers to the concentration of the stock or ‘initial’ solution, and V 1 refers to the volume of the initial solution. C2 refers to the concentration of the final solution, and V 2 refers to the volume of the final solution. Example Using a 2M stock solution of NaOH prepare 500 mL of a 0.125 M solution C1 = 2M C2 = 0.125 M V1 = ? V2 = 500 mL Rearrange the formula to make V1 the subject V1 = C2V2 ie V1 = 0.125 M x 500mL C1 2M V1 = 31.3 mL Hence if 31.3 mL of 2 M NaOH is diluted to 500 mL a solution of 0.125M will be made. The units on both sides of the equation must be the same. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 50 Laboratory Techniques for Environmental Technicians Exercises 1. A stock solution of 0.750 M NaCl is available. What volume, in mL, is required to make 100.0 mL of 0.10 M? Type your answer here 2. Concentrated H2SO4 is 18.0 M. What volume is required to prepare 2.00L of a 1.00M solution? Type your answer here 3. Concentrated HCl is approximately 11.8 M. What volume is required to prepare 2.0L of a 1.0M solution? Type your answer here 4. A 0.500 M solution is to be diluted to 500 mL with a final concentration of 0.150 moles/L. What volume of the stock solution is required? Type your answer here 5. A stock solution of 10.0 M NaOH is prepared. From this solution you need to make 250.0 mL of 0.375 M solution. How many mL will be required? Type your answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 51 Laboratory Techniques for Environmental Technicians Practical: Metal colours in a flame Background: Atoms when excited by flame, electric current etc are known to become excited. When this occurs electrons jump to higher energy levels and on cooling fall back into vacant electron holes. This results in energy, often in the form of light being emitted. The colours emitted can be a useful identification tool. Purpose: To observe the colours of various metal ions in solution Procedure: (This will be done at the intensive session) Aspirate each of the known metal ion solutions into the flame and note the colour of the flame. Aspirate the unknown metal solutions and identify from the colour of the flame the metal in solution Results: Metal ion Flame colour Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 52 Laboratory Techniques for Environmental Technicians Chapter 3: Safety in the Laboratory Safety in the Laboratory Purpose: This section is designed to provide a brief overview of work safety in the laboratory. You will need to refer to the text for laboratory safety for additional information. Work health and safety laws have greatly improved safety and wellbeing in the workplace. All employers must provide safe and healthy work conditions, all workers must work within the safety systems and both groups must accept responsibility for identifying and controlling hazards and minimising risk of harm. These laws are continually being reviewed and the current Work Health and Safety Act 2011 resulted in a change from 1 January 2012. Any laboratory has a range of hazards that need to be controlled to minimise the risk of harm. A working laboratory is really not much different to working in your home kitchen! What is a hazard? A hazard is something that can cause harm. For example a Lion is a hazard as it could kill a person What is risk? Risk is the potential (likelihood) for a hazard to cause harm. For example, if your were a lion tamer the risk of the lion would be high. However, if you were in a laboratory where there are no lions the risk would be low. What does hazard control mean? This means that after identifying the hazard and the risk that appropriate control measures are used to either eliminate the hazard or reduce the risk. Hazard control uses a principle known as the Hierarchy or Control in order to control an identified hazard Explain the hierarchy of control Type your answer here Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 53 Laboratory Techniques for Environmental Technicians Laboratory hazards Physical Chemical Biological Electrical Explosives Infective agents – bacteria, viruses, etc Mechanical Corrosives Animal and plant toxins Manual handling Flammable gases Allergens Cuts Flammable liquids Sensitisers Burns Flammable solids Biological active substances Radiation Radioactivity Answer Compressed gases Oxidisers Answer Mixes of the above Answer Dust Mixes with other types of hazards Answer Confined space Poisons Answer Stress Noise Note that there are acute vs chronic issues, dose and Answer response issues, exposure standards, monitoring / Answer awareness problems Light Answer Of the above laboratory hazards are there any that also apply to your kitchen? Use an asterisk * to indicate those that also apply to your home. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 54 Laboratory Techniques for Environmental Technicians All chemicals should be considered to be dangerous because they may be: ◗ toxic / poisonous : damage body organs or tissue by interfering with the normal chemical processes occurring in those tissues ◗ irritating: cause a bad response to eyes, skin and other organs ◗ flammable : burn or cause heat damage to tissue ◗ explosive: create a shock wave of high speed and high pressure that causes mechanical damage ◗ corrosive : chemically degrades tissue at the point of contact. Specific organs such as lungs, liver, eyes and skin may be affected or gross damage can occur at all points of contact. Damage can range from: ◗ slight tissue damage or irritation to total destruction ◗ minor, temporary change to widespread permanent damage ◗ immediate evidence of symptoms to long-term delay before onset. ◗ Effects may be acute (quick acting) or chronic ( build up over time). A chemical is only dangerous if it enters your body. This can only occur by: ◗ inhalation ◗ absorption ◗ ingestion ◗ injection What controls would exist for each of these chemical hazards in a laboratory? Type your answer here The law (Work Health & Safety 2011) now requires information sources about hazardous chemicals to be supplied. They are called Safety Data sheets (SDS) and they help you to find out about dangerous properties of chemicals and what measures to take to reduce harmful effects. Typically a SDS provides information about: ◗ common names and identification codes for the material ◗ physical properties ◗ major hazards of the material ◗ acute and chronic symptoms of exposure ◗ exposure standards ◗ medical advice ◗ spill and other emergency responses ◗ others Search the ‘Web” for an SDS for concentrated hydrochloric acid and identify information relating to the hazardous nature of the material. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 55 Laboratory Techniques for Environmental Technicians Laboratory rules and regulations ◗ Always wash your hands before you leave the laboratory ◗ Only supervised work is permitted in the laboratory ◗ Chemicals and equipment are not to be taken from the laboratory ◗ Safety glasses, laboratory coats and appropriate shoes must be worn in the laboratory at all times ◗ Clean-up spills immediately ◗ Act responsibly - the laboratory is not a playground, racetrack or amusement parlour ◗ Long hair must be tied back ◗ You must be aware of the location and operation of safety equipment ◗ All accidents and incidents must be reported ◗ Consult the SDS for unfamiliar chemicals ◗ Spillage of any chemical on the skin or eyes should be immediately treated with copious quantities of water and the supervisor’s attention sought ◗ Eating and drinking in the laboratory is banned ◗ Fume cupboards should be used for work involving dangerous gases or vapours The Laboratory is a safe place to work if you follow the rules Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 56 Laboratory Techniques for Environmental Technicians Practical 1.1 Laboratory Layout Date completed: _____________________ Teacher Check _______________ Purpose Analyst signature ____________ To become familiar with the layout of the work area, in particular, those areas and pieces of equipment that deal with safety. Procedure: Draw a map of the laboratory, which shows the location of the following features: Fire extinguishers, fire exits, fire control equipment; special storage cupboards, safety showers, eye wash stations, first-aid, fume cupboards, antidotes, laboratory store, ovens, balances, waste disposal facilities. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 57 Laboratory Techniques for Environmental Technicians Clean-up of spills Common spills which can occur in the laboratory include solid reagents, solutions of all kinds, various organic solvents and special ‘nasties’ such as mercury. All spills are to be treated according to the immediate dangers. For example it may be necessary to: ◗ evacuate (e.g. poisonous gas or vapours) ◗ ventilate (e.g. organic solvent fumes) ◗ isolate services (e.g. flooding around electrical services) ◗ contain (e.g. prevent spread of fluid or other material beyond the site of the spill) ◗ absorb (e.g. use an inert absorbent to soak up a spill) ◗ neutralise (e.g. concentrated acids and bases need to be neutralised first to minimise corrosion to the cleaning equipment and the cleaner) ◗ clean up (e.g. sweep up the solid residues) ◗ dispose (e.g. may need a clean-up service because of the hazardous wastes) ◗ rehabilitate (e.g. surfaces may have been damaged and will require refinishing). The Golden rules for personnel safety in the laboratory: ◗ Leave the laboratory clean and tidy ◗ Put your equipment away ◗ Clean up all your mess ◗ Keep your equipment and community property clean and well maintained ◗ If you borrow anything, return it ◗ Any safety and hazardous situations or issues should be drawn to the supervisor’s attention. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 58 Laboratory Techniques for Environmental Technicians Practical 1.5 Safety in the laboratory Date Completed: _____________________ Teacher Check ______________ Procedure: Analyst signature ____________ Observe the demonstrations on safety in the laboratory Give a brief description of the demonstration List the observations you made during the class and in the next laboratory session Comment on aspects of the demonstration that you found interesting, disturbing, informative etc Results: Test/ Demonstration Observation Hydrochloric acid Session 1 Comments HCl + Meat Session 2 Sulfuric acid Session 1 H2SO4 + Meat Session 2 Session 1 Nitric Acid HNO3 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 59 Laboratory Techniques for Environmental Technicians + Session 2 Meat Session 1 Sodium Hydroxide NaOH + Session 2 Meat Session 1 Ammonium hydroxide NH4OH + Session 2 Meat Session 1 Propanone Acetone CH3COCH3 + Meat Session 2 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 60 Laboratory Techniques for Environmental Technicians Session 1 Session 2 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 61 Laboratory Techniques for Environmental Technicians Dangerous goods 'Class' labels Class 1 - Explosives Class 2 - Gases Class 3 - Flammable liquids Class 4 - Flammable solids Class 5 - Oxidisers Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 62 Laboratory Techniques for Environmental Technicians Class 6 - Toxic and infectious substances Class 7 - Radioactive material Class 8 - Corrosives substances Class 9 - Miscellaneous dangerous goods Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 63 Laboratory Techniques for Environmental Technicians Chapter 4: Basic Laboratory Equipment Basic Laboratory Equipment Laboratory testing is used to answer at least one of these basic questions: ◗ “what is this material?” ◗ “what are the components in this material?” ◗ “how much of each component is in this material?” In other words, the lab can analyse a sample to describe what it is or what is in it –is there: ◗ mercury in this fish? ◗ salmonella in this chicken meat? ◗ cocaine in this powder? ◗ alcohol present on this person's breath? The lab can analyse a sample to describe how much of any component is in it: ◗ how much mercury in this fish? ◗ how much salmonella in this chicken meat? ◗ how much cocaine in this powder? ◗ how much alcohol present on this person's breath? The ‘what” questions are questions about quality and such laboratory work is called qualitative analysis. (if you answer 'yes' to the 'what' questions above, what does it tell you about quality of each sample?). Qualitative analysis requires qualitative equipment and the major focus is on isolating and purifying components and identifying components. Accuracy in measuring mass and volume is not a big deal because we are not answering anything about ‘how much’. It is quite acceptable to use measuring cylinders and 2- and 3decimal place balances. Accuracy in measuring physical properties, eg RI, pH, m. pt., etc is very important because we use these numbers to decide on purity and identity. The ‘how much” question is a quantity question and such laboratory work is called quantitative analysis Equipment for Handling chemicals must be chosen considering the purpose required: ◗ storage or ◗ transfer or ◗ processing or ◗ measurement Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 64 Laboratory Techniques for Environmental Technicians You will work with many types of equipment in the intensive session. The following is just some of the equipment commonly found in a general ‘wet’ laboratory. Storage containers For large volumes: ◗ aspirators ◗ drums ◗ Winchester bottles (2.5L) For smaller volumes and also for transfer: ◗ Conical flasks ◗ Beakers ◗ Conical beakers Measuring glassware (both quantitative and qualitative): ◗ Measuring cylinders ◗ Pipettes ◗ Burettes ◗ Volumetric flasks Miscellaneous equipment: ◗ Spatulas ◗ Funnels ◗ Evaporating basins ◗ Test tubes ◗ Mortar and pestle ◗ Tongs ◗ Balances ◗ Weigh boats ◗ Bunsen burners ◗ Heating mantles ◗ Desiccator ◗ Thermometers ◗ Retort stands ◗ Condensers ◗ Various hoses and tubing Do you know any more to add to the list? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 65 Laboratory Techniques for Environmental Technicians The following table lists basic apparatus used in the laboratory. Complete the table by giving a use for the equipment and any safety features that need to be considered. Diagram Name Use / Safety Test tube Measuring cylinder Watchglass Graduated and bulb pipette Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 66 Laboratory Techniques for Environmental Technicians Diagram Name Use / Safety Burette Beaker Conical flask Evaporating basin ` Crucible Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 67 Laboratory Techniques for Environmental Technicians Diagram Name Use / Safety Pipe clay triangle Tripod Gauze mat Volumetric flask Filter funnel Buchner funnel Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 68 Laboratory Techniques for Environmental Technicians Diagram Name Use / Safety Vacuum flask Spatula Dropper Tongs Condenser Mortar and pestle Desiccator Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 69 Laboratory Techniques for Environmental Technicians Chapter 5: Introduction to Material Handling In any laboratory, certain techniques are regularly performed and are essential to most routine work. These techniques include the measurement and use of chemicals and other hazardous substances and the operation of laboratory equipment. A competent technician uses these basic techniques, confidently and without risk, in routine work and with any new procedures. Procedures include: ◗ material transfer ◗ weighing ◗ volume measurement ◗ solution preparation ◗ filtration ◗ heating, cooling and drying ◗ temperature measurement ◗ using gas cylinders ◗ particle size reduction ◗ cleaning and drying glassware. Material transfer Materials are routinely moved from one container to another during laboratory work. The transfer usually has to meet at least one of the following requirements: ◗ safety with materials which are toxic, corrosive or dangerous in some way. ◗ quantitative transfer where any loss will affect the accuracy of the task. ◗ contamination control either from the surroundings to the material or vice versa Solid transfer ◗ usually handled by a spatula or weighing vessel or in the original container. ◗ If the solid is to be made up into solution then a solvent may be used to assist the transfer ◗ The transfer must always be quantitative Liquid transfer ◗ Generally liquids pour well ◗ A small amount of residue may be left in the container and depending on the use of the liquid this may need to be washed into the next container ◗ Volumetric glassware has very specific requirements for transfer and these will be discussed later. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 70 Laboratory Techniques for Environmental Technicians Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 71 Laboratory Techniques for Environmental Technicians Practical work 5.1 Basic material handling techniques Date Completed: ______________________ Teacher check ________________ Purpose: Analyst signature ____________ All chemicals whether solid, liquid or gas must be handled with caution. This practical looks at the techniques to be employed when transferring solids and liquids. Procedure: Observe the demonstrations of material transfer shown by your trainer. Identify the mistakes and indicate how the process should be completed. Procedure Errors Corrections Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 72 Laboratory Techniques for Environmental Technicians Volume measurement techniques Good laboratory practice aims to: ◗ control spills and dribbles ◗ avoid contact with hazardous contents ◗ reading the scales on the glassware from the meniscus location ◗ obtain quantitative measurement, transfer and delivery without loss or error ◗ make the correct choice of quantitative and qualitative glassware ◗ develop a sense of how valid a transfer or a measurement has been. Liquid Transfer Techniques Transferring the liquid to a new container (receiver) needs to avoid: ◗ spillages (missing the receiver) ◗ dribbles (some liquid runs down the outside of the original container) ◗ losses (not all of the liquid reaches the receiver). Reading the Meniscus Measuring liquid volume requires you to read the position of the meniscus on a scale. The meniscus is the curvature or shape adopted by a liquid surface near the walls of any container. It can curve up or down. For example, mercury in glass, is concave down whereas water in glass, is concave up. All volume judgments are made by comparing the flattest portion of the meniscus with the scale on the vessel. With water this becomes the bottom of the meniscus and with mercury it is the top. The volume scale may be a single mark (pipette, volumetric flask) or a continuous scale (burette, graduated pipette, measuring cylinder) which can cause trouble if the meniscus lies between two markings. You should only guess to half of the smallest division on the scale. Accurate and Approximate Volume Measurement When accuracy in volume measurement is the first concern, then volumetric glassware (quantitative glassware) must be used with the correct technique and the right attitude. The professionalism which tells an analyst whether a volume measurement has been performed validly or not, comes from: Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 73 Laboratory Techniques for Environmental Technicians ◗ proper understanding ◗ technical skills ◗ the correct attitude. In particular an attitude of near enough is good enough is not acceptable. Sometimes the analyst knows the volume measurement or transfer has been faulty and must make the professional decision to discard the faulty material and repeat the necessary steps. The word aliquot is commonly used to denote an accurately measured volume of liquid and will be used frequently in this text in the section on titration. For those volume measurements where the amount needed is not critical, the following may be appropriate or convenient: ◗ a qualitative glassware such as measuring cylinders ◗ graduated beakers or flasks ◗ any of a wide range of droppers fitted with a suction bulb (e.g. Pasteur pipettes, droppers fitted to reagent bottles and even droppers fitted with graduated stems) ◗ an experienced guess. Nevertheless, you should still pay attention to safety and proper transfer technique and avoid parallax errors. Types of droppers Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 74 Laboratory Techniques for Environmental Technicians Pipetting Techniques All single-mark pipettes are used by filling them to an upper mark. A sacrificial rinse (three times) with the fill solution is the minimum effort all users must make to ensure the pipette is clean. This must be done even if the pipette is dry or after normal cleaning. The sacrificial rinse only removes fresh contaminants or solvents which may have entered during current usage. The entire contents are then delivered to the receiving vessel General pipette rules Perform sacrificial rinse with the fill solution. Overfill to above the mark. Set the bottom of the meniscus on the line. Allow to drain in a vertical position until no more liquid runs out. Touch the tip on the side of the receiver to draw the final amount of liquid out. Do not blow it out. There must always be a little left behind, which is allowed for in the original calibration. Overfilled Too low Meniscus is spot-on The pipette is not yet empty Too high The pipette has been properly emptied Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 75 Laboratory Techniques for Environmental Technicians Burette Techniques All burettes have a highly graduated scale which starts from 0.00 mL at the top and goes down to the biggest volume at the bottom. A sacrificial rinse with the fill solution is the minimum effort all users must make to ensure the burette is clean. This must be done even if the burette is dry or after normal cleaning. The sacrificial rinse only removes fresh contaminants or solvents which may have entered during current usage. It is normal to use a funnel to fill burettes but the funnel must be removed before zeroing the burette as liquid is often trapped between the funnel and the burette and tends to escape half-way through a job and ruin the measurement in progress. Burettes can have a range of taps at the bottom, from the traditional glass stopcock (which needs a touch of lubricant and care not to pull it out as it is being used), to more modern teflon stopcocks and rotoflow-type screw taps which are much easier to use, do not come out easily and are self-lubricating. Techniques for using burettes are shown in Figure 5.5. General burette rules ◗ Perform sacrificial rinse with the fill solution. ◗ Overfill to above the mark (zero or any other). ◗ Open the tap fully and ensure all air bubbles are driven out of the tap and the tip of the burette. ◗ Set the bottom of the meniscus on the chosen mark — usually zero. ◗ Clamp in a vertical position at a convenient height above the receiver. ◗ Touch a wastes receiver onto the side of the burette tip to draw any excess liquid off the tip. ◗ Check your mark and record its value. ◗ Run the required volume of liquid out and ensure any suspended drop on the burette tip is also transferred. ◗ Read and record the new volume. Perform any necessary subtractions. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 76 Laboratory Techniques for Environmental Technicians Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 77 Laboratory Techniques for Environmental Technicians Volumetric Flask Techniques All volumetric flasks have a single mark and hence contain but a single known volume and all are used by filling them to this mark. A sacrificial rinse with the intended solvent is the minimum effort all users must make to ensure the volumetric flask is clean. This must be done even if the flask is dry after normal cleaning. The sacrificial rinse only removes fresh contaminants or solvents which may have entered during current usage. The primary purpose of volumetric flasks is to make up solutions or dilutions of solutions. General volumetric flask rules ◗ Perform sacrificial rinse using intended solvent. ◗ Add the solute first, quantitatively, without loss. For many solutes, the transfer into a small beaker for dissolving in the solvent is a useful technique to avoid loss of solute. The solute may be solid or liquid or a solution already. ◗ Add some solvent to ensure the solute dissolves completely. ◗ Transfer carefully with pouring and rinsing to the volumetric flask ◗ Mix well by inversion and swirling. ◗ Solvent is added until the bottom of the meniscus is on the line — not over, not under — but on the line. Mix well and support the weight correctly. ◗ Label with name, concentration, date and appropriate safety advice. You cannot remove any liquid if you overshoot the line when making up to volume. You will also remove an unknown amount of solute and hence invalidate the job. The last step requires a final mixing which is done by inversion and swirling, using the trapped air bubble to ensure mixing occurs. The lid and flask bottom must be solidly supported or a spill or a breakage will result. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 78 Laboratory Techniques for Environmental Technicians When the lid is removed to access the contents, you must take care to avoid contamination of the contents by the laboratory environment and vice versa from a carelessly placed lid. Measurement Of Mass Mass in the everyday world is measured on scales such as those used for people or vegetables or fertiliser. Bigger versions are used to check trucks or bales of wool and smaller versions are used by the jewellery trade. In the scientific world, the term ‘scales’ is replaced by the term ‘balance’ when describing tools for the measurement of mass. Laboratory Weighing Weighing of materials is fundamental to most procedures. You should be competent at working with each of the balances found in the general laboratory. Weighing rules ◗ all balances must be clean, level and zeroed ◗ all chemicals must be weighed by difference in a container ◗ don’t weigh hot or wet objects ◗ clean up all spills immediately ◗ check the balance has returned to zero and reset it before you leave. Common terms used with weighing or the use of a balance include: ◗ clean: nothing on the balance pan ◗ level: the workbench and the balance need to be horizontal ◗ balance reading: the mass on display ◗ true mass: the mass of the sample after the container mass has been subtracted ◗ reset: leave the balance clean, level and zeroed ◗ weighing by difference: an empty container is weighed, the sample is added to it and the mass of the container and material recorded, the difference between the final mass and the empty container is the mass of the material ◗ taring: the balance pan is adjusted to read zero with the empty container sitting on the balance pan, when the material to be weighed is added, the displayed weight is the weight of the added material only Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 79 Laboratory Techniques for Environmental Technicians Practical work 5.2A Introductory weighing task Date Completed: ______________________ Teacher check ________________ Purpose Analyst signature ____________ The aim of the practical is to become familiar with the different types of balances available in a general laboratory. Your instructor will demonstrate the correct procedures to follow when using a balance. Procedure: Use the range of laboratory balances supplied as shown during your teacher’s demonstrations. You will perform repeated measurements which will be used to check your accuracy and that of the balances. Record the capacity and sensitivity of the balances. Results: Balance Object Code ........ Object Code........ Object Code ........ Triple Beam: Capacity: ........... Sensitivity;.......... 3 dp top pan Capacity: ........... Sensitivity .......... 4 dp analytical Capacity ............ Sensitivity ......... Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 80 Laboratory Techniques for Environmental Technicians Other Basic Laboratory Procedures Separation of one component from another in a mixture can be a routinely necessary task in a laboratory. Filtration is used to separate an insoluble solid from a liquid. The procedure requires the use of a barrier called the filter, which is permeable to the liquid but retains the solid. The liquid portion is termed the filtrate or mother liquor and the solid is the filter cake or residue. Two common approaches to filtration are: ◗ gravity ◗ vacuum assisted Gravity filtration is a simple method of separating a solid from a liquid. It involves the use of a filter paper, available in various pore size and diameter, folded and placed in a filter funnel. The mixture is poured in the top, the paper allows the liquid to pass and the residue is left behind. Vacuum filtration is used where large quantities of solids and/or fluids are involved and fast separation is required. Problems arise if the solid is very fine or gelatinous. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 81 Laboratory Techniques for Environmental Technicians Practical work 5.5A Simple filtration Date Completed: ______________________ Teacher check ________________ Purpose: To become familiar with simple filtration techniques, following instructions and noting safety requirements. Analyst signature ____________ Note: The chemicals in this practical are very toxic. Ensure that any spills are reported and also ensure that hands are properly washed if skin contact occurs and before leaving the laboratory. Record the identity and quantity of each of the chemicals Dispense an aliquot for each reagent bottle into a clean dry beaker Carefully mix, using a glass stirring rod, the beaker contents over a steam bath for five minutes Cool the beaker in an ice bath Set up a filtration apparatus as shown by your teacher and using a labelled, pre-weighed filter paper filter your sample. Wash the filter cake well with distilled water Transfer the filter cake and filter paper to a labelled watch-glass and allow to dry Reweigh and determine the amount of solid material. Results: Identity of solution 1 Volume of solution 1 used Identify of solution 2 Volume of solution 2 used Mass of empty filter paper Mass of filter paper + precipitate Mass of precipitate Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 82 Laboratory Techniques for Environmental Technicians Questions: When is filtration a suitable method of separation? One of the solutions you worked with contained lead. Why is it necessary to be extremely careful when working with this chemical? How would you find out the necessary safety requirements for working with lead solutions? Why was it necessary to stir the solution over a water bath? Why was it necessary to allow the solution to cool before filtration? When filtering the solution is not filled to the top of the filter paper, why? Draw a diagram to represent the simple filtration apparatus. List all the possible places where losses may have occurred. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 83 Laboratory Techniques for Environmental Technicians Heating in the laboratory A laboratory also will have an array of heating, cooling and drying equipment such as gas burners, heating mantles, muffle furnaces, hot plates, steam baths, ovens, desiccators and refrigerators. Common gas burners include the bunsen, the meker, batswing and microburner. These burners all depend on a supply of fuel gas and air. The efficiency and heat output of any burner can be controlled by selecting the amount of air and/or gas which passes through the burner . Bunsen burner Batswing burner Meker burner Your teacher will demonstrate the correct ignition procedure for the common laboratory burners . Generally if you are going to leave a bunsen burner alight, but not in use, the yellow “safety” flame should be burning. Non-flame devices are versatile and much safer for use with flammable materials. These include electric hotplates, drying ovens, muffle furnaces, heating mantles and drying cabinets. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 84 Laboratory Techniques for Environmental Technicians Practical: 4.4 Heating devices Date Completed: ______________________ Teacher check ________________ Procedure: Analyst signature ____________ Examine the heating equipment on display and complete the following table. Device Description Major Use Safety Hazards Flame dependent devices (Burners) Bunsen Meker Batswing Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 85 Laboratory Techniques for Environmental Technicians Microburner non – devices flame Heating mantle Electric hot plate Muffle furnace Laboratory drying oven Heat lamp Steam / water / sand baths Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 86 Laboratory Techniques for Environmental Technicians Others Practical: Application of heating equipment Date Completed: ______________________ Teacher check ________________ Procedure: Follow the instructions for each task and record the data where required. Analyst signature ____________ Task 1 Unscrew the stack on each of the supplied burners and examine the features of each burner. Note the gas jet or nipple and the rotatable sleeve which admits air and mixes it with the gas as they moves up the stack. Attempt to light the gas at the nipple. Note - the gas supply may need to be very low for this. Replace the stack and light the Bunsen as demonstrated by your teacher. Note the effect of sleeve positioning on the appearance of the flame. Using the blue flame, turn the gas pressure down slowly so that the flame gets smaller and strikes back down the stack to burn at the gas nipple. This condition is extremely dangerous because: the Bunsen appears to be off and hence is a fire hazard the stack will be heated by the flame and will burn anyone who touches it 6. Repeat the above five steps with the Meker and micro-burners. Draw and label a Bunsen and discuss your observations below Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 87 Laboratory Techniques for Environmental Technicians Task 2 Use the supplied thermocouple to measure the temperature of various zones in both the ‘blue’ and ‘yellow’ versions of the Bunsen flame. Complete the following table: Zone of the flame Yellow Flame Blue Flame Diagram to locate each zone being measured temperature temperature (OC) (OC) Approx. 20cm above the tip of the flame At the tip of the flame 20 cm In the heart of the flame (top of blue cone) Level with stack opening the Task 3 This section is designed to investigate the changes which occur to the gas mixture in the flame. Following the demonstrations given by your teacher, examine the presence of zones of unburnt gases in a Bunsen burner as follows: Use a thin glass tube inserted directly into the bottom of the blue cone to tap off some of its contents – attempt to ignite the gases at the other end of the tube Pierce a live match with a pin inserted at right angles approximately half way along the match – suspend this match with its head pointing up, on the stack of an unlit Bunsen. Light the Bunsen, with the air hole open. Observations Record your observations and provide an explanation of what is happening. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 88 Laboratory Techniques for Environmental Technicians Chapter 6 The dreaded calculations This chapter has an online attachment that you may find useful for the calculations. The assessment for the unit is composed of 2 open book assignments that are located in the appendix of this theory booklet. Laboratory Data and Error Analysis How can you know if you have the right answer? Unfortunately, there is often no simple way to know whether the correct answer has been obtained. To improve our confidence, scientists have developed ways to estimate the uncertainty in the measured value(s) and how they differ from the true or correct answer(s). Laboratory scientists use a series of terms when describing uncertainty and errors. Accuracy A measurement is accurate if the correct answer was obtained. The correctness of an answer depends on what standards have been set (i.e. how close to the true answer is acceptable). Hence every measurement will have some degree of accuracy and also a level of error. Scientists also talk of accurate instruments or inaccurate methods because of the quality of the instrument’s design or the limitations of the method. Error The amount by which the true value has been missed is called the error. Some errors are brought about by the limitations of the equipment. Analysis of these limitations can be used to calculate the size of the errors. Other errors are linked to limitations in the skill or vigilance of the operator or shortcomings in the method. These errors are much more difficult to identify and compensate for. Precision If very similar answers (a standard for closeness needs to be set) are obtained for a set of measurements (e.g. duplicate, triplicate, replicate determinations), the answers have a high level of precision. Precision can refer to the results of a person or a testing method or a laboratory. Measurements which lead to answers which are highly precise, are often taken as evidence that the answers are also accurate. However if the same error was committed each time, good precision may still be all wrong. Reliability Repeated performance of a procedure which achieves good results every time, means it is reliable and its errors are under control. This is used to describe the quality of a technician or a measurement or a technique or a method. Reliability is also known as repeatability. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 89 Laboratory Techniques for Environmental Technicians Validity A method or procedure must be able to be supported by traditional scientific reasoning as well as the good data it produces. For example, a number of alternative healthcare practices exist which are labelled as invalid because traditional practitioners can’t see a scientific link between the alternative approach and current understanding of medicine. The improvements in the patient can’t be taken as proof of the validity of the alternative approach. Classification of Errors All scientific work is subject to errors and the skill of a competent worker is to understand where errors may come from and how to minimise or compensate for them. Errors normally can only arise from defects in: ◗ the operator ◗ the equipment ◗ the method being followed. Operator errors are those errors for which the operator is responsible and can be caused by physical handicaps (e.g. colour blindness), bias, prejudice or poor attitude to quality and accuracy. Examples of operator errors include: ◗ mechanical loss or gain of materials during weighing, filtering, liquid transfer ◗ failure to obey essential conditions such as temperatures, times or conditions ◗ incorrect performance of required techniques such as dilution, titration, instrument optimisation and operation. Equipment errors are due to defects in the analyst’s tools and equipment or the effects of environmental factors upon them. Common equipment errors are: ◗ random defects such as a balance or oven not operating as expected ◗ systematic defects producing results which appear acceptable but are consistently in error; they are caused by the use of tools such as weights, graduated glassware and thermometers which are out of calibration ◗ equipment which is not appropriate for the task; you needed to use a different size or model or system ◗ reaction of reagents and samples with glassware and other containers, resulting in the presence of interfering materials ◗ use of reagents containing impurities. Method errors are due to errors in the procedure or the technique. Common method errors are: inaccuracy — the method used is not adequate to get the measurement needed; measurement of pH using indicators or test strips may be inaccurate because the conditions in your sample interfere with the performance of the indicators Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 90 Laboratory Techniques for Environmental Technicians lack of validity — the method cannot cope with limitations of your sample; moisture in many fresh foods cannot be validly obtained by simple oven drying; lead in blood or pesticides in meat require extremely sensitive testing equipment and cannot be done by simple test-tube reactions calculations and processing of data which have been incorrectly carried out incorrect sampling or sample preparation failure of the necessary reactions to go to completion occurrence of side reactions and by-products. Another system uses the terms systematic (or determinate) errors and random (or indeterminate) errors to classify the errors which affect an experimental result. Systematic errors are those that have a definite value which can be measured and accounted for, that is, they are errors that are possible to avoid, minimise or compensate for. Random errors result from the person, the equipment or the method operating outside its limitations. These errors may or may not be positively identified and so will not always have a definite measurable value. Random errors occur in a random manner. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 91 Laboratory Techniques for Environmental Technicians Systematic and random errors can be minimised by: ◗ calibration of apparatus and application of correction factors ◗ use of blanks, spiked samples, controls, certified reference materials, recovery tests and variations in sample size ◗ alternate or independent methods of analysis ◗ replicate determinations ◗ standard addition techniques ◗ internal standard techniques ◗ validation using the literature or prior work with similar samples Basic Numerical Methods for Reporting Measurements When any scientific measurement is carried out, it is not enough to simply carry out one analysis, and to report the result. There are too many sources of possible error that can arise, and therefore the analysis should be repeated a number of times to confirm that the measurement obtained is reliable. The result that is reported after several, acceptable, measurements of the same property is the average of the repeats or the mean. This is calculated from the sum of the answers divided by the number of values. For example, an analyst determines the vitamin C content of a sample and she obtains the following results: 24.39 mg/L, 24.18 mg/L, 24.27 mg/L. The reported value for this analysis would be the mean of these determinations, that is: ( 24.39 + 24.18 + 24.27 ) / 3 = 24.28 mg/L. The two attributes needed to accept these data are: accuracy (i.e. the result should be as close as possible to the actual value) precision (i.e. the replicate values should be close to each other). In this case, if the accuracy could not be estimated, the precision would be considered good and the analyst should feel confident with her work. When accuracy estimates are essential (e.g. nutritional labelling, medical analysis), then a number of validation methods exist. Routine validation methods which could be used are: control samples — samples for which the amount of analyte (vitamin C) is already known; the analysis for these must be correct to be able to accept the others spiked samples — samples which have already been analysed and to which a known amount of analyte (vitamin C) has been added; the increased level of analyte allows a new expected result to be calculated and again the analysis for these spiked samples must be correct to be able to accept the others alternative methods or procedures — if the same answer is still obtained then both approaches would be valid. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 92 Laboratory Techniques for Environmental Technicians Relative and Absolute Descriptions of Error The absolute error (or absolute accuracy) is the difference between the observed value and the true value. The relative error (or relative accuracy) is the absolute error expressed as a percentage of the accepted value. The sign of the error may be positive or negative, indicating that the result is high or low respectively. The absolute precision is half of the range of the measurements. The relative precision is the absolute precision expressed as a percentage of the mean of the measurements. These definitions are summarised in the Table below Definitions and formulae used to describe errors Symbol Interpretation Formula X A measured or observed value x1 , x2, x3, etc. for all your readings R The range from biggest to smallest of all replicates for this measurement R = x biggest – x smallest µ The average of all replicates for this measurement µ = [x1 + x2 + x3 + … ] number of replicates X true The true or correct value Eabs The absolute error or accuracy Eabs = X – Xtrue or Eabs = µ – Xtrue Erel The relative error or accuracy Erel = Eabs × 100 Xtrue Pabs The absolute precision Pabs = Prel The relative precision Prel R 2 = Pabs × 100 µ Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 93 Laboratory Techniques for Environmental Technicians Errors and Limits of Reading The uncertainty of any reading is half the value of the smallest scale division. Thus if your smallest scale division is one unit your answer can be shown as 0.5 unit. Reading scales and assigning uncertainty Read the scales below and write down your readings for the position of the pointer in each case. There are no units required. Assign an uncertainty figure to each of your scale readings. Use after the value for each reading. a b h n o c d e i p j q k r f l g m s t and u (a) (h) (o) (b) (i) (p) (c) (j) (q) (d) (k) (r) (e) (l) (s) (f) (m )(n) (t) (g) (u) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 94 Laboratory Techniques for Environmental Technicians Chapter 7: The why’s of sampling Introduction Sampling is a process where portions of a material are taken for testing. The test results are then assumed to apply to the entire material. Scientific testing absolutely depends on linking the laboratory results for a sample to the bulk of the material from which it came. If there is no valid link, there is no point in the test. How would you, as a science technician, advise authorities about a testing system for the analysis of drinking water from a river or dam? Three different approaches or options would cover your choices: Option 1 — test the water while it is still in the dam Option 2 — take some water out and test it with field equipment Option 3 — take some water out and send it to a laboratory. Option 1 seems good because the water is undoubtedly representative and it is hard to see it being changed by the test process. For example a pH probe or a dissolved oxygen sensor can be dipped directly into the water from a boat and readings taken from as many different sites as is needed. Option 2 could be necessary when some sample treatment like heating, filtering and other chemical additions are necessary. But the danger exists that the sampling will take a bad batch of water or contaminate the water which is taken and so the test results may not validly apply to the rest of the dam. But what other choice do you have? Option 3 would be needed when field equipment can’t do the job and the controlled environment of a laboratory is needed. The problems now include the time and conditions which may cause the sample to undergo significant physical, chemical and biological changes. Oils and greases may be lost on the walls and lid of the container, bacteria may multiply or die, fine sediments may settle and absorb heavy metals or pesticides. Sounds risky? In reality, all approaches are used and all are satisfactory so long as the limitations are understood and sampling is carried out properly. The best equipment, technical expertise and hard work cannot compensate for a poor sample. Sampling Requirements Some materials are very easy to sample without bias because they are very uniform. Others are more complex which makes sampling very difficult to perform correctly. Liquids such as: ◗ drinking water ◗ milk Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 95 Laboratory Techniques for Environmental Technicians ◗ blood ◗ diesel fuel would be tested using a sample which was only a tiny fraction of the whole, and the results would be very confidently applied to the entire bulk of the material. Uniform or homogeneous materials are usually free of bias in sampling (but are still liable to problems with dirty containers and poor storage conditions). When all portions of a bulk material are not identical (the material is heterogenous), it becomes possible to collect biased samples. The earlier liquid examples could become: ◗ muddy water ◗ curdled milk ◗ clotted blood ◗ diesel fuel floating on a layer of water. and so the sampler needs to know whether to skim some good stuff off the top or just grab a bucketful and hope for the best. Obviously the law does not allow us to just hope for the best when human health and safety are at risk and a better system is needed. In general, the better system has been developed after much trial and error. Gases are usually well mixed (e.g. atmospheric and flue gas samples which are moving) but layers can develop in confined spaces such as tanks and mines, where different gas composition may build up on the floor or ceiling. Again, the sampling techniques will depend on the uniformity expected in the bulk material. Experience, technical skills and statistical competency are all needed to get it right. Solid materials of natural origin (e.g. coal, agricultural produce and minerals) may or may not be uniform and synthetic products such as building materials, processed foods, cosmetic preparations and cleaning agents can have many different components blended into a number of different phases and at varying concentrations. Sampling of these for testing in order to meet the legal and quality and economic standards of the organisation is of great importance to future survival and prosperity. A final example to introduce the needs and difficulties of sampling is taken from an area of global concern — our environment. The build-up of harmful contaminants and of dangerous toxins in our air, water and soil is well known. Responsibility and control is difficult to enforce because it is hard to prove: how much pollution existed before (background levels of carbon dioxide, ozone, radioactivity, heavy metals and coal and petroleum by-products) who released the contaminants, since they quickly become dispersed or diluted or mixed in with those from next door. Unless you catch them, it is hard to allocate blame correctly. Sampling of contaminated and uncontaminated sites is a necessary step to answering such questions, but how to perform the process to clearly identify the source is not easy. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 96 Laboratory Techniques for Environmental Technicians Sampling Terminology A sample is a small portion of a large mass of material and must be representative of that mass. The mass could be hundreds of tonnes of ore, coal or grain; thousands of litres of liquid such as milk or petrol, or millions of cubic metres of natural gas, or even the whole atmosphere. Quite clearly, analysis of the small portion should give an answer for the concentration of each analyte to accurately reflect its concentration in the large mass which it represents. A representative sample must be identical in its chemical and physical characteristics to the whole. Thus it must be taken correctly and stored and processed for testing in such a way that its representative status is not affected. A specimen refers to a portion or single part of the whole. It may or may not be representative of the whole. Hence a nice specimen of coal can be picked up from a stockpile because of appearance or size or convenience, but this alone does not guarantee that it is a representative sample of this coal stockpile. Homogeneity refers to the degree of uniformity in the composition and distribution of each analyte in the bulk material. Homogeneous materials are entirely uniform and any specimen is also a representative sample. Heterogeneous materials lack uniformity in their overall bulk in at least one property (colour, particle size, chemical composition, hardness, crystallinity, etc.) and a specimen may not be representative. Well-mixed gases and liquids are considered to be homogeneous. Conditions are possible however, where gases and liquids can form layers of different composition due to: vapours and evaporation (e.g. petrol) phase separation (e.g. silt in river water) temperature (e.g. cooking oils) corrosion effects in containers (e.g. confined spaces and oxygen depletion). Solids can be homogeneous or heterogeneous depending on the component of interest. Cement looks to be a uniform grey powder to the casual observer, but particle size distribution is critical to its performance and it is considered to be heterogeneous. It requires a rigorous sampling scheme to detect particle size violations. Potatoes on the other hand look very heterogeneous yet potato chip manufacturers would not care too much about size, shape or colour so long as they all contained very similar amounts of reducing sugars to ensure they cook to the same final golden colour at the end. Coal is particularly complex with a range of classification schemes linked to the end use (steam generation or coke production). Strict specifications on the range of physical and chemical properties are Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 97 Laboratory Techniques for Environmental Technicians set by buyers and ultimately these specifications dictate the price of the coal. Proper sampling and testing of coal is critical to the commercial success of any coal supplier. Random sampling suggests that the sampler should gather material in a widely distributed pattern but the pattern should not bias the removal to only one particular type of material (e.g. the loose bits on the floor that would otherwise need to be swept up). The design of the sampling pattern is critical to the confidence levels of the overall findings. Each industry needs to carefully design its random sampling scheme and samplers need to understand the effects of departure from this scheme. Bulk (or gross) sample describes the end result of the collection of material in a sampling program. The size of this sample can be quite large (hundreds of kilograms) and often needs to be subsampled to obtain laboratory samples. Subsampling refers to the process used to reduce the size of a sample in a representative manner so as to obtain a more convenient quantity for laboratory work or storage. A variety of manual (cone and quarter) and mechanical (riffle, sample splitter) methods are available to ensure that this is done representatively. These are described later on. A laboratory sample is the portion of the bulk sample provided to the laboratory for its testing purposes. It has strict labelling requirements to ensure it can be linked to the original supply. It must be preserved to ensure its properties are not changed by storage or handling. An analytical sample is that portion of the laboratory sample which is actually tested. It still needs to be representative and also may have pretreatment requirements in terms of its physical and chemical state. Sample preparation is a description of the later stages of sample handling. Typically this may require grinding or milling, sieving, drying, filtering, cooling and a variety of other chemical and physical treatments. The figure below shows an idealised relationship between your bulk supply and the sample tested by the laboratory. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 98 Laboratory Techniques for Environmental Technicians Samples taken to represent the bulk supply A bulk supply of material can have the following variations: identity size state uniformity danger accessibility value A laboratory sample of adequate size for testing and back-up Analytical samples which are labelled and preserved for testing and storage Relationship of bulk supply to laboratory sample Steps in sampling Collect the gross or bulk sample from the material stockpile. Homogeneity of solid bulk samples can be improved by coning (see Figure 6.3). Reduction of the gross sample to a convenient size for laboratory handling. This is done by coning and quartering, rifling, tabling, sample splitting, etc. (see Section 6.5). Preparation of sample for analysis. Repeated coning to homogenise a bulk solid sample Obtain your individual samples form the composite into a cone Combine individual samples into the composite sample Take away the first cone by the edges and make a new cone continue until you form a new cone and repeat the coning process until you have a satisfactory homogenate Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 99 Laboratory Techniques for Environmental Technicians The sampling process can present a number of dangers. There may be: ◗ physical dangers from the location (height, temperature, confined space) ◗ dangerous behaviour of the bulk material (unstable stockpile, dusts, volatility) ◗ hazards because of its chemical properties. The sampler must wear adequate protective clothing, have a knowledge of the material being sampled, and follow standard procedures laid down for the sampling programme. ◗ Sampling Equipment and Techniques ◗ Solids in general require a sequence of steps to be followed: ◗ collect the gross sample by random sampling, ◗ sub-sample the gross sample to create the laboratory sample, ◗ prepare the analytical sample by appropriate pre-treatment before analysis. Large samples can be manually reduced into smaller representative portions by riffles, coning and quartering, rolling and quartering, and other forms of sample splitting. Some solids such as metals need to be drilled to obtain suitable samples and soil sampling in the field may need core samples to be taken. Surface samples such as chips, clods and shavings may not be representative. A series of chutes or slides to split and deliver the gross sample to a A riffle box set of new locations or collection points Gross sample is poured into the chute system at the top Other chute patterns Sub samples emerge and are collected at the bottom of each chute. Riffle designs used to sub-sample the gross sample Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 100 Laboratory Techniques for Environmental Technicians You have a laboratory sample of a suitable size Or using a technique of coning and quartering to reduce the sample size Figure 6.5 The quartering process used to subsample the gross sample Liquids which are static and homogeneous are very easy to sample as any small portion will represent the whole. A sample may be obtained by from a single point (called a grab sample) using a container made from material appropriate for storage of that liquid. Liquids with concentration gradients, separate phases and suspended or filtrable solids need more thought, planning and equipment. These are normally sampled using devices such as diptubes, depth sampling bottles and sample thieves (see Figures 6.2 and 6.6). Piped liquids which are sampled through outlet valves, can be extremely dangerous because of the pressure and temperature of the emerging fluid. Gases which may be sampled could be: ◗ toxic, explosive or flammable ◗ hot or cold ◗ high or low pressure ◗ contaminated with moisture or particulates ◗ unstable and require special preservation (eg. environmental air pollutants). Many can be collected under their own pressure, but some sampling situations may require a pump. Some gases are deemed to be of such concern that they are analysed by continuous on-line equipment which automatically collects its own sample. For example, air in underground mines can be explosive or poisonous or depleted in oxygen and needs Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 101 Laboratory Techniques for Environmental Technicians constant careful monitoring. This equipment needs to be calibrated periodically for the quantity of material taken and the response of the sensor system. Portable gas testers such as stain tube analysers (also called Draeger™ tubes) and the explosimeter for flammable gases are excellent field instruments, but some gases need to be collected, often over an extended time period, by an absorbing medium (charcoal or reactive liquid medium) for later analysis. Sample Preparation, Preservation and Labelling Some samples, even if taken in a valid and representative way, may still not lead to an accurate analysis of the bulk material because of a number of difficulties. For example: The sample may change or no longer represent the original, because conditions in the sample are no longer the same as the bulk material. Examples include:: food taken from a hot or cold storage area (and so moisture levels, bacterial growth, vitamin levels may be different), a water sample from a pool or river or dam (a different temperature, dissolved oxygen and light level will affect some components), a blood sample (taken for blood alcohol or other component remains static but the person’s biochemistry continues to function) or a soil coring (drying out, contact with air leads to chemical and physical degradation). The sample may have a chemical or biological composition which protects an important component from being detected accurately. Silicates are notoriously insoluble and samples with silica content need to undergo severe grinding and digestion procedures to ensure their components are released for analysis. The sample has chemical or biological components which are particularly sensitive and need to be protected in some way. The sample may need to be stored for a long period as a backup or because of delays in analysis. The only answer to all these difficulties is to apply a scientific approach to the perceived problems. Each individual case needs to be assessed for where it may be breaking down, if it can be fixed by reasonable means, and if you are able to report a valid upper or lower limit for the analysis. Standard methods exist to address sample preservation and sample preparation. Sample preservation or protection methods include: ◗ cooling eg. food and biological samples ◗ acidification eg. heavy metals in water samples ◗ thiosufate additions eg. destroys chlorine to protect micro-organisms in water samples ◗ sterile containers and aseptic handling techniques Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 102 Laboratory Techniques for Environmental Technicians Sample preparation is the procedure followed in treating the sampled material so it is suitable for testing in the laboratory. The following steps may occur: grinding, milling or blending as a way of reducing particle size and ensuring the necessary components are released from the material digestion where the sample is treated with acids or buffers or other solutions to ensure the release of desired components in their correct chemical forms for analysis. Labelling is the final requirement of the sampling program and needs to identify the following essentials: ◗ date ◗ identity of the sampler and bulk sample ◗ the destination of the sample ◗ its testing requirements ◗ the destination of the analytical results. Often there are a series of codes for these. Computerised systems are becoming routine for reporting results. They ensure that sample results are properly logged, checked for accuracy, and accounts and reports produced and despatched. Sometimes a reserve sample is put into storage in the event of a dispute. Its labelling must allow it to be identified as part of the original sample. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 103 Laboratory Techniques for Environmental Technicians Practical: Date Completed: Validation of sampling ___________________ Teacher check _____________ Purpose Analyst signature ___________ This practical task is designed to demonstrate whether the sampling procedure used has produced a sample that truly reflects the composition of the bulk material. You will create a bulk supply of material of known composition (~5% salt in sand) and you will sample it for laboratory testing. You will measure its true salt content and compare your answers to the expected values. Procedure Mix sand (500 g) and sodium chloride (25 g) together to make a homogeneous blend by coning on a sheet of plastic. Record the masses used in your logbook. Sample your mixture by the cone and quarter technique until you have a laboratory sample of approximately 25 g for testing. Weigh three 5 g samples into 100 mL beakers for analysis. Record the actual masses in the table below. Analysis of samples To each sample in a beaker, add distilled water (50 mL), mix well to dissolve the salt and filter through a preweighed filter paper into a preweighed evaporating basin. For each sample, use another portion of water (20 mL) to rinse the remaining salt residues through the sand. Allow both washings to combine. Evaporate the liquid in the evaporating basin on a steam bath. It may be necessary to complete the drying in an oven at 105C. For each filtrate, obtain the dry mass of salt and record it in your logbook. Dry each sand sample in an oven until it is moisture-free (constant mass). Record each sand mass in your logbook. Results Bulk Supply Mass of sand Mass of salt (g) Sand + salt % sand (g) total (g) % salt Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 104 Laboratory Techniques for Environmental Technicians Sampling details Laboratory samples Mass empty beaker of Mass beaker sample of Mass of laboratory Mass of analytical + sample sample used in test 1 1. 2. 3. Analysis details for analytical samples Mass empty paper of Mass filter paper sand of Mass of Mass of basin Mass check on + empty + salt residue cleaned out empty evaporating basin basin 1 2 3 Analysis calculations Mass of sand Mass of salt Recovered Original mass of % sand recovered (a) recovered sand + salt analytical Recovered (b) total (a) + (b) sample used in test % salt Recovered 1 2 3 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 105 Laboratory Techniques for Environmental Technicians Questions: Discuss any differences between the composition of the original bulk supply and the test results for your recovered samples. Comment on your recovery check. (the agreement between the mass of each of your analytical samples and the recovered sand + salt masses after the analysis. This tells how reliable your results might be. Suggest how you could improve the method to achieve better % composition and recovery check agreement. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 106 Laboratory Techniques for Environmental Technicians Practical: Date Completed: Sampling Equipment ___________________ Teacher check _____________ Analyst signature ___________ Purpose This practical is designed to familiarise the technician with equipment available to assist in the taking of a valid sample. Procedure ◗ Record the sample identity which you have been allocated by the tide zone from which it was obtained. ◗ Using the riffles provided reduce the sample to approximately 100g and record the analytical sample size in the table provided. ◗ Repeat step 2 ◗ Select a nest of sieves and clean them thoroughly as demonstrated by the teacher, . ◗ Record the aperture sizes and assemble them so that the aperture decreases from biggest at the top to the smallest next to the catch pan. ◗ Transfer one of the analytical samples to the top of the nest of sieves. ◗ Shake the sieves (with the lid on) for 5 minutes ◗ Using the A3 paper method demonstrated by the teacher, carefully capture and record the mass of each fraction. ◗ Repeat the procedure with the other samples. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 107 Laboratory Techniques for Environmental Technicians Results: Sample number Sample mass location on the beach from which the sample was taken: 1 2 Sieve size Mass sand Sample 1 % sand fraction in Mass sand Sample 2 % sand fraction in Total mass Questions: Did you recover 100% of the initial sample? If not where did you gain or lose sample in the method? Did you have good agreement between your triplicate samples? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 108 Laboratory Techniques for Environmental Technicians Chapter 8: Solution Preparation General volumetric flask rules ( A recap) ◗ perform a sacrificial rinse using the intended solvent ◗ add the solute first, quantitatively, without loss ◗ add some solvent to ensure the solute dissolves completely ◗ mix well by inversion and swirling ◗ solvent is added until the bottom of the meniscus is on the line, mix well and support the weight correctly ◗ label with name, concentration, date and appropriate safety advice. 1. Add solute without loss 2. Dissolve in some solvent 3. Invert and swirl to mix 4. Make up to the mark and repeat 3. Steps to make up a solution in a volumetric flask Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 109 Laboratory Techniques for Environmental Technicians Practical 5.4A Introductory Solution Preparation Date Completed: ______________________ Teacher check ________________ Analyst signature ____________ Procedure: You are required to prepare and validate known concentration solutions. Using an analytical balance weigh out accurately the mass of each solute indicated in the result sheet table. Quantitatively transfer the solid to a 100mL volumetric flask and make up to the mark with distilled water The teacher will show you the checks to be made on your sample and the previously prepared sample Results: Potassium Hydrogen Phthalate (Labelled as KHP) mass = 1.0 ±0.1g Your Solution readings Standard Sample readings Sample mass: Sample volume: pH reading Conductivity Potassium chloride (labelled as KCl) mass = 5.0 ± 0.5g Your Solution readings Standard Sample readings Sample mass: Sample volume: pH reading Conductivity Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 110 Laboratory Techniques for Environmental Technicians Questions: Why is it important that an analytical balance is used to measure the mass? Why is it important that the transfer of solid material is quantitative? Why was a volumetric flasks specified in each case? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 111 Laboratory Techniques for Environmental Technicians Chapter 9: Basic environmental laboratory testing Laboratory Measurements The following physical properties can be measured easily in the laboratory; ◗ pH ◗ refractive index ◗ density ◗ conductivity ◗ melting point ◗ boiling point pH Measurement of pH is a widely applied technique for monitoring agricultural, industrial and biomedical processes. pH is a measure of the acidity of a sample, a low pH (ie <7) indicates the sample is acidic, a high pH (>7) indicates the sample is basic. pH may be measured using: ◗ indicator papers: paper impregnated with coloured dyes, whose colour is sensitive to the pH of the solution to which they are exposed ◗ indicator solutions: solutions which are made up from the same coloured dyes which are used for indicator papers. They are added directly to the test material where the coloured form is used to assign a pH value. pH meter: an electronic instrument which gives a direct readout of pH. It uses a glass probe which generates an electrical signal in proportion to the hydrogen ion concentration of the solution in which it is immersed. The pH meter must be calibrated prior to use to ensure the validity of the result. Practical work 7.1 pH measurements Date Completed: ______________________ Teacher check ________________ Analyst signature ____________ Procedure: The practical tasks provide experience with various detection methods for the determination of pH. Your teacher will demonstrate the use of each method. Whilst working with each consider the accuracy and efficiency of the method. Indicator solutions, test papers and the pH meter are used to measure pH. Different requirements for accuracy and speed will dictate which method is appropriate. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 112 Laboratory Techniques for Environmental Technicians Complete the table below by determining the pH of the samples provided, using the methods as shown by your teacherResults: Sample Litmus Red Litmus BT Blue B Initial colour Phen olphtha lein Univer sal indicat or Othe r? Dip stick pH meter Questions: Which method do you consider the most reliable? Which household chemical was the most acidic? Which household chemical was the most alkaline? What information does litmus paper give? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 113 Laboratory Techniques for Environmental Technicians Measurement of density The density of any gas, liquid or solid is its mass per unit volume. mass Density= volume Mass is measured with a balance Volume is measured by direct reading of a volume scale on the container or from displacement measurements by immersion in a suitable liquid. The object is immersed in the liquid and the apparent rise in level equals the volume of the object. Problems arise when: ◗ the material is soluble ◗ the void spaces absorb fluid ◗ the material dissolves, swells, expands or shrinks Liquid density measurements Two basic methods are used ◗ density bottles (pycnometers): highly precise volumetric vessels which are filled with the liquid in question and weighed. ◗ hydrometers: specially weighted floats which are immersed in the liquid. Refractive index can be correlated with density. The producers and users of sugar-based materials make extensive use of density as measured by hydrometry and refractometry to analyse for sugar content. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 114 Laboratory Techniques for Environmental Technicians Practical: 7.7 Density Date Completed: ______________________ Teacher check ________________ Analyst signature ____________ Procedure: The practical is designed to give the student skills in determining the density of a variety of different types of material, (irregular solids, granular solids, liquids etc) using a variety of techniques (hydrometry, pycnometer etc). Density of an irregular solid ◗ obtain three objects and record their identity ◗ measure and record the mass of the objects ◗ partially fill an appropriate measuring cylinder with water and record the initial volume ◗ Carefully immerse the object in the measuring cylinder ◗ record the new volume (final volume) ◗ Calculate the true volume ◗ complete the table by determining the density Sample Identity or code mass (g) Initial Volume (mL) Final Volume (mL) True Volume (mL) Density (g/mL) Literature Density You need to reference the source of your literature value data here. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 115 Laboratory Techniques for Environmental Technicians 2. Bulk density of various granular solids ◗ obtain two different granular solids ◗ record the mass of an appropriately sized dry measuring cylinder ◗ add a predetermined volume of sample to the measuring cylinder and record this volume in the table as the initial volume ◗ reweigh the measuring cylinder, record the mass ◗ gently tap the measuring cylinder to pack down the granular solid ◗ record the compacted (settled) volume ◗ add a known quantity of water to the measuring cylinder ◗ record the new volume in the measuring cylinder ◗ calculate the true volume of the granular solid ◗ complete the worksheet Sample I.D. Empty cylinder mass (g) Sample + Cylinder Mass sample (g) Initial Sample volume Settled volume Volume water added Final volume Mass (g) Sample ID True volume of Bulk Density sample Settled density True Density Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 116 Laboratory Techniques for Environmental Technicians 3. Density of liquids ◗ weigh and record the mass of a clean, dry 25mL measuring cylinder ◗ add 25 mL of the liquid sample to the measuring cylinder ◗ reweigh the measuring cylinder and record the mass ◗ complete the worksheet ◗ follow the teacher’s instructions regarding disposal of the organic solvents Sample I.D. Empty cylinder mass (g) Sample + Sample mass (g) cylinder mass (g) Volume sample (mL) Density (g/mL) Lit. Density Questions How well do your practical results compare to the literature values? What errors are present in the procedure you followed that would decrease your accuracy? Identify the chemicals that would be of concern in the workplace. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 117 Laboratory Techniques for Environmental Technicians 4. Density of liquids by hydrometry ◗ follow the teachers instructions regarding use of the hydrometer ◗ carefully float the appropriate hydrometer in a measuring cylinder of the allocated liquid ◗ read the value from the bottom of the meniscus Sample Hydrometer Literature value How well do your practical results compare to the literature values? What errors are present in the procedure you followed that would decrease your accuracy? Of the two methods (3 and 4) which do you prefer and why? 5.Determination of sugar content by hydrometry and refractometry carefully measure the hydrometer reading of each of the sugar solutions provided, ensuring that the hydrometer is dried before placement in a new solution carefully measure the refractive index of each of the solutions following instructions given by the teacher determine the concentration of sugar in each of the unknowns by plotting i) Hydrometer reading vs sugar content ii) Refractive index vs sugar content Sample identity Hydrometer reading Refractive index 5% sugar 10% sugar 15 % sugar 20% sugar unk 1 unk2 You must plot a calibration graph (manually or by excel) for your RI and hydrometer results and use it to calculate the sugar content of each of your unknowns. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 118 Laboratory Techniques for Environmental Technicians Chapter 10: Gravimetric analysis Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a : ◗ loss of volatiles procedure where the sample is heated to release volatiles such as moisture or organic vapours and the change in mass is used to calculate the volatile content, or ◗ precipitation or separation procedure where a component in the sample is isolated or recovered in some form and this is weighed to complete the analysis. Loss of volatiles gravimetric analysis All loss of volatiles analysis is performed by a similar set of simple steps: ◗ weigh your fresh sample ◗ heat to remove volatiles ◗ reweigh to measure mass loss ◗ calculate percentage loss of mass or percentage residual mass In practice, many problems can arise if you consider what might go wrong in each of the steps. You need to: ◗ weigh enough sample to keep accuracy high but not too much so as to hinder vapour loss ◗ heat but not for too long or too hot or too cold ◗ cool without additional losses or pick-up of volatiles ◗ reweigh without losses or gains ◗ be sure the container does not change weight during the procedure. Heating to constant weight is the chief method used to ensure the container is not altered during treatment. Empty containers are subjected to the same treatment as the sample before any analysis and if their masses remain constant, then you can be sure they have been heated to constant weight and their empty mass is correct. Precipitation or separation gravimetric analysis Precipitation or separation gravimetric analysis follows these simplified steps: ◗ weigh your fresh sample ◗ treat to isolate component of interest ◗ measure mass of pure, recovered material ◗ calculate percentage of component Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 119 Laboratory Techniques for Environmental Technicians The treatment required to isolate the component can cover a wide range of techniques and procedures including: ◗ digestion with powerful reagents such as enzymes, acids or oxidants ◗ buffering to ensure conditions are suitable for recovery of the analyte ◗ addition of chemicals designed to selectively and quantitatively capture the component of interest. ◗ use of prolonged heating or contact with solvents such as occurs with Soxhlet extraction or Dean and Stark moisture determination. Validation of Gravimetric Analysis A requirement with all laboratory testing is some evidence or assurance of the reliability of your answer. This evidence comes in four major ways: ◗ replicate analysis ◗ control or standard analysis ◗ literature values ◗ alternative testing procedure Common containers for gravimetry Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 120 Laboratory Techniques for Environmental Technicians Practical: Analysis of a mercury chloride and determination of its empirical formula. Date Completed: ___________________ Teacher check _____________ Analyst signature __________ Introduction In this practical you will be given a compound of mercury and chlorine and by decomposing the compound into its elements you will be able to calculate the percentage composition and the empirical formula of the original compound. Decomposition of the mercury chloride is achieved by heating the substance with a mixture of sodium hypophosphite and hydrochloric acid until the element mercury is produced. WARNING All compounds of mercury should be considered to be highly poisonous- don’t breathe in the fumes from the solution and make sure that the mercury is covered by solution during heating. Wash your hands thoroughly at the completion of the exercise and make sure all spillages are cleaned up correctly! Procedure: ◗ Obtain a sample tube containing a mercury chloride and record the number on the tube. ◗ Weigh a clean, dry evaporating basin, labelled with your name. Also weigh the sample tube plus contents ◗ Carefully add the mercury chloride to the evaporating basin. ◗ Collect 20 mL of distilled water and use some to rinse the contents of the sample tube into the evaporating basin. Add the remainder of the water to the evaporating basin. Reweigh the empty, dry sample tube. ◗ Add 10 mL of sodium hypophosphite/hydrochloric acid solution from the dispenser bottle to the contents of the evaporating basin. ◗ Place over a steam bath in a fume cupboard and stir to mix the reactants (Care - liquid is corrosive) ◗ Continue heating and stirring until mercury collects in large silvery globules. (Avoid inhaling vapour while heating) ◗ When all the mercury globules have coalesced, remove the basin from the steam bath and decant the colourless liquid AS DEMONSTRATED BY THE TEACHER. ◗ Wash the contents of the basin twice with water and once with small amounts of methylated spirits. Collect all washings in the waste container provided. ◗ Dry the mercury by pulling pieces of filter paper through it as demonstrated. ◗ Re-weigh the basin containing the dry mercury. ◗ Return the mercury to the class collection bottle. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 121 Laboratory Techniques for Environmental Technicians Results Sample ID Mass evaporating basin Mass tube + contents Mass empty tube (after washing) Mass evaporating basin + mercury Calculations Calculate by subtraction of appropriate measurements ◗ Mass of mercury chloride used ◗ Mass of mercury metal produced ◗ Mass of chlorine originally present From these figures calculate a) the percentage composition of your compound b) the empirical formula of your compound c) suggest a suitable name for your compound Discussion Comment on any errors in your analysis and how this may be overcome. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 122 Laboratory Techniques for Environmental Technicians Practical: Gravimetric determination of sulfate in bore water 8.6 Date Completed: ___________________ Teacher check _____________ Analyst signature __________ Purpose To determine the sulphate content of bore water using a precipitation method. Procedure ◗ Accurately weigh approximately 2.og of fertiliser and transfer to a 500mL beaker. Record the mass labelled on the beaker in the Results section ◗ Add approx 50 mL 0.1M hydrochloric acid from the dispenser ◗ Gently heat the beaker to boiling on a hot plate to dissolve the fertilizer (there will be some undissolved material). ◗ While the heating is proceeding – ◗ From the desiccator weigh a #4 sintered glass crucible. Do NOT touch the glass surface with bare hands, use tongs to avoid contamination. Record the Empty mass in the result table. ◗ Assemble a vacuum filtration set up utilizing a Buchner funnel (fit with drip tip towards the vacuum spout) and an appropriate sized type 54 filter paper. ◗ When the fertilizer solution boils remove the beaker from the heat and allow it to cool before filtering. (Use an ice bath to cool if necessary). ◗ Filter the fertilizer solution. Rinse the beaker 3 times with distilled water to ensure all the solution passes into the vacuum flask. ◗ Discard the filtered-out insolubles (on the filter paper) and return the filtrate (the liquid) to the original labelled beaker, again using 3 small distilled water rinses. ◗ Add 25 mL (by measuring cylinder) of Barium Chloride to the beaker. The reaction converts the soluble sulfate ions to insoluble Barium Sulfate. Ba2+(aq) + SO42+(aq) BaSO4(s) ◗ Return the beaker to the hot plate, cover with a large watch glass and heat gently (no boiling) for about 1 hour, then allow to cool. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 123 Laboratory Techniques for Environmental Technicians Fit the pre-weighed sintered glass crucible to your vacuum filtration set up. (No touching with fingers!) Use the sintered glass crucible to filter the Barium Sulfate crystals. After transferring all the contents of the beaker wash the crystals 3 times with about 10 mL of purified water each time. Wash the inner wall of the sintered glass crucible and the crystals with ethanol. Leave under vacuum for about 1 minute, this will ‘vacuum dry’ the crucible and crystals. Transfer the crucible into a 110° drying oven. Weigh the crucible with its load of dry BaSO4 crystals (no touching!). Record the mass in the table . Results Sample 1 Sample 2 Crucible ID Mass of empty crucible (g) Mass of empty crucible (g) after heating to constant weight Mass of crucible + residue (g) Mass of crucible + residue (g) at constant weight Mass of residue (g) Average mass of residue (g) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 124 Laboratory Techniques for Environmental Technicians Calculations The value reported is typically in the units of mg sulphate / L Sulfate = average mass of residue x 40 x 0.4113 x 1000 Questions What is a supernatant solution? What is the purpose of adding more BaCl2 to the supernatant fertilizer solution? What is happening to the crystals while they are kept in the hot solution for an hour? Why were the Barium Sulfate crystals washed repeatedly? Barium Chloride crystals have 2 molecules of water associated with each molecule of BaCl2 ie. BaCl2•2H2O How does this affect the way you would prepare a 5% solution of BaCl2? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 125 Laboratory Techniques for Environmental Technicians Practical: Experimental Investigation of combustion of magnesium An open-ended investigation is one in which the student is asked to investigate a particular problem, with no specific information being provided about the end result. The student is asked to consider the possibilities and make judgements on how to proceed. In this practical you are to investigate the combustion of magnesium. A method will be provided but before commencement you are being asked to decide what will happen to the magnesium, will it change in any way, will it look the same, how might it change, will it be the same after as it was before…… Write your answers to the above before commencement of the practical Combustion of magnesium When magnesium is burnt (combusted) in air, it reacts with the oxygen gas forming a white ash of magnesium oxide. This is a highly exothermic reaction producing a large amount of energy as heat and light. Equation Magnesium(s) + oxygen(s) Magnesium oxide(s) + energy This practical investigates the mass of magnesium compared to the mass of the magnesium oxide (ash) produced. In an enclosed vessel (crucible) the magnesium will quickly use up available oxygen as it burns and so we will need to open the lid several times to allow air to enter. If sufficient oxygen is not present the nitrogen (from the air) will often combine with the magnesium forming white magnesium nitride and so the ash contains both magnesium compounds. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 126 Laboratory Techniques for Environmental Technicians Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine how the mass of magnesium burnt compares with the mass of the ash formed Procedure Weigh an empty clean crucible with its lid and record the mass Place three 2 cm pieces of magnesium ribbon in the empty crucible and record the mass Heat the crucible, with the lid slightly ajar, in a strong Bunsen flame After several minutes check that the magnesium has been burnt. Allow the crucible to cool to room temperature with the lid on. Weigh the crucible with contents and lid and record the mass Results Mass of crucible + lid = Mass of crucible + lid + magnesium = Mass of magnesium Mass of crucible + lid + ash = = Mass of ash = Conclusions The law of conservation of mass (matter) states that in any chemical reaction the total mass of reactants is equal to the total mass of products since the reaction involves just the rearranging of the atoms which are already present “Matter can neither be created nor destroyed, it can only be changed form one form into another” Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 127 Laboratory Techniques for Environmental Technicians Questions Assuming that your magnesium totally reacted to produce magnesium oxide, what mass of oxygen must have been used from the air? When a piece of wood burns the resulting ash produced weighs much less than the original wood. Where did all the other atoms go? The main elements in wood are carbon, hydrogen and oxygen. What compounds do you think carbon and hydrogen would form when the wood is burnt in air? Carbon would combine with oxygen to form ………………………………. Hydrogen would combine with oxygen to form ……………………………. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 128 Laboratory Techniques for Environmental Technicians Practical: Gravimetric determination of Ni by precipitation with dimethylglyoxime. This method involves converting nickel ions which are soluble in water into a complex which has reduced solubility at an increased pH. The procedure involves the use of a complexing agent dimethylglyoxime and strict pH monitoring to form an insoluble solid. The complex formed is Nickel dimethylglyoxime. The analysis is gravimetric as the precipitate formed is collected and weighed to determine the mass of nickel. Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine the nickel content of a water solution SAFETY The technique involves digesting the solution over a steam bath. Remember steam and skin do not go well together. Ammonia has a strong odour and work with ammonia should be carried out in the fume hood. When smelling do not inhale deeply (Use the wafting technique as demonstrated by your teacher.) Concentrated HCl and skin to not go well together. The sintered glass crucibles will be hot when removed from the drying oven Procedure Clean two sintered glass filter crucibles with dilute HCl and dry in a 110 oC oven. Pipette out two 20 mL samples of the supplied unknown into two 600 mL beakers Dilute to about 150 mL with purified water Add approximately 3g of citric acid to each solution and stir to dissolve. Leave the glass stirring rod in the beaker Slide a piece of red litmus paper down the side of the beaker until it is mostly immersed in the solution, but sticks to the side. Add 5 M ammonia slowly until the litmus turns blue, and then add concentrated HCl slowly until the paper returns to red. Add 2 mL more of concentrated HCl. Remove the litmus paper. Heat the beakers on a hotplate to about 80oC but do not allow to boil. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 129 Laboratory Techniques for Environmental Technicians Remove the beakers from the hot plate and slowly add 50 mL 1% dimethylglyoxime (DMG) solution with stirring. If a red precipitate begins to form, add concentrated HCl dropwise until it redissolves, and then continue to add the DMG solution. Add 5M ammonia slowly with stirring until a red precipitate forms and the solution smells strongly of ammonia. Stir well and stand on a steam bath for 30 minutes. Check that the solution still smells of ammonia, (add extra if necessary) Add 2 mL extra DMG using a plastic pipette, and stir well. Cool the beakers to room temperature and filter the solutions through the previously dried and tared sintered glass filter funnels. Wash the precipitate in the crucible 3 to times with water Finally wash the precipitate once with 20-30 mL of 30% aqueous ethanol, which dissolves any remaining DMG from the precipitate. Place the crucibles in an oven at 110oC for at least 2 hours. The crucibles should be cooled in a desiccator and then reweighed. Results Sample 1 Sample 2 Volume solution taken Mass of crucible sintered glass Mass crucible + Ni(DMG)2 Calculations 1. Ni2+(aq) + 2DMG Ni(DMG)2(s) Moles Ni2+ in original solution = mass Ni(DMG)2 = moles of Ni(DMG)2 formed . 288.88 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 130 Laboratory Techniques for Environmental Technicians Mass nickel in original solution = moles of nickel x Formula mass nickel Sample 1 = Sample 2 = Ave = %w/v of nickel in the sample = mass of nickel in sample x 100 vol of sample QUESTIONS Why was it important to limit the amount of nickel in the sample by only taking 20 mL? Why was the precipitate washed in aqueous ethanol? Why was a sintered glass crucible used rather than filtration through paper? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 131 Laboratory Techniques for Environmental Technicians Chapter 11: Volumetric Analysis This section builds on the chemistry in chapter 2 and the theory is provided only as a means of illustrating the concept. It does not form an examinable part of the units being undertaken in the intensive session. It is the titration skill that will need to meet the required level of competence. The titration process The terms volumetric analysis, titrimetry and titration are used interchangeably to describe a procedure which analyses chemicals in solution by accurate volume measurement. Principles of titration Titration is used to determine the concentration of an analyte in solution (the unknown solution) and hence the components of any material which can be made up into solution may be analysed this way. The method operates by reacting the analyte in the unknown solution with another solution (the standard solution). The process is carried out so that reaction between the two can be stopped exactly at the point when the last trace of analyte has been used up. The volume of both standard and unknown are used to obtain the required quantitative information. To perform titrimetry, the following need to be available: ◗ standard solutions ◗ volumetric glassware ◗ a suitable method of detecting the end-point ◗ a minimum quantity of analyte ◗ a standard that reacts appropriately with your analyte The burette is filled with a known concentration of chemical R which destroys A An accurately measured amount of the material containing analyte (A) R is added until the very last of A is destroyed – but not one drop more of R is allowed An aliquot of the is put into a known volume of test solution is solution now measured for titrimetric analysis Only A This flask now contains a fixed number of particles of A A is being consumed The amount of A is calculated from the amount of R which was added. Both A and R no longer exist by R as it is added Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 132 Laboratory Techniques for Environmental Technicians The practical term, end point, is used to describe the volume of standard solution which was used to just consume the aliquot of analyte. The theoretical term, equivalence point, is used to describe the volume needed to provide the exact molar quantity for the analyte to react completely with the standard. The end point is usually a little more because you need to have a little extra to cause the indicator to change colour. Theoretical Requirements Titrations require the following conditions: a rapid and complete chemical reaction between the analyte and the standard. Those reactions which are sufficiently fast and complete to be suitable for volumetric analysis include acid-base, redox, complexation and precipitation reactions. This enables an extraordinary number of analytes to be tested by titration and this versatility makes the procedure so universally popular. Some titrations are carried out at higher temperatures to ensure the reaction is rapid and complete. a balanced equation for the reaction. To be able to calculate the final answer, you must know the molar ratio of reaction between the analyte and the standard and that the chemical change has gone to completion. a means for detecting the disappearance of the last trace of analyte. Because the volume of addition is judged by the disappearance of analyte, a signal which is linked to this event is mandatory. This signal can be a colour change which is detected by eye (or some other property such as a temperature or conductivity change, which is detected instrumentally). The most common methods use indicator solutions which are coloured dyes with two coloured forms — one colour exists while analyte is still present and the other colour is created when the analyte disappears. The analyst performing the procedure needs to be alert to detect the first permanent colour change. The addition is stopped and the volume of titrant (solution added from a burette) is measured. The titration is said to be at its end point and this volume becomes one of your measured end-point volumes. Practical Requirements To perform titrimetry, the following need to be available: standard solutions (solutions of exactly known concentration). These should have a reasonable shelf life and be easy to prepare from ingredients that are convenient to handle. A more stringent set of requirements appears in Section 9.3 on types of standards. volumetric glassware (burette, pipettes and volumetric flasks) and support equipment a suitable method of detecting the end point a minimum quantity of analyte. Below this minimum, the method will not be able to detect the presence and then the absence of analyte reliably. Typically, titration can not reliably measure concentrations of less than 0.1%, unless some form of pre-concentration is used Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 133 Laboratory Techniques for Environmental Technicians a standard that reacts appropriately with your analyte. You can’t just take any standard solution and do a titration and expect a valid result. There must be a known reaction and indicator that detects a true end point. Not all analytes have appropriate standards for titrimetry. Two analytes for which titrimetry does not work are nitrate ions, and fat in food. Selection of Indicators Indicators are coloured chemicals which have two forms — each with a different colour. The chemical form and colour of the dye are affected by the chemical conditions in the solution in which it is dissolved. Ideally, an indicator is selected because the presence of the analyte favours one coloured form and the absence of analyte favours the other. From the practical viewpoint, catalogues of indicators reveal there are scores of choices and so a selection procedure is required. Initially you might suspect that each analyte requires its own indicator and any indicator can only identify one analyte, but this is not true. The choice of the correct indicator is determined by the conditions at the end point or equivalence point. Indicators are classified as: ◗ acid-base ◗ redox ◗ adsorbtion ◗ or complexometric and must match the type of reaction occurring during titration. All indicators have precisely defined conditions for changing colours. For example, the key factor for acid-base indicators is pH at the end point. Table 9.1 shows some common acidbase indicators, their colours, and their pH changeover range. The names of indicators are deceptively simple and easily confused (e.g. methyl violet is not even violet). Many have similar colours (e.g. yellow and red), but change at different pH intervals. The direction of change may also be reversed (e.g. methyl orange is red at low pH and yellow otherwise, whereas phenol red is yellow at low pH and red otherwise). Acid–base indicators Indicator pH changeover high pH colour range low pH colour Methyl violet 0.0–1.6 blue yellow Methyl orange 3.2–4.4 yellow red Phenol red 6.6–8.0 red yellow Bromothymol blue 6.0–7.6 blue yellow Phenolphthalein 8.2–10 pink colourless Alizarin yellow R 10–12 red yellow Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 134 Laboratory Techniques for Environmental Technicians End point pH is determined by the strength (not concentration) of acid and base used, and the natural pH at the equivalence point when exact molar proportions are present for all the acid to neutralise all the base. Weak acid–strong base equivalence points (e.g. ethanoic acid–sodium hydroxide) are all greater than pH 7. Strong acid–weak base equivalence points (e.g. HCl–Na2CO3) are all less than pH 7. Your procedure needs to have information on end-point pH or a specified indicator. The Titration Procedure The diagram below shows the general equipment used for titration. A burette is used to contain one of the solutions (either the standard or the analyte solution) and a conical flask or conical beaker holds the other. The following steps are then performed. Burette Rinse the burette with a small amount of the fill solution as the final step before filling. Obviously if there is any doubt, a more thorough cleaning will be required prior to the final filling step. (Remember the ‘magic number’ three) Overfill with the required solution. Check there are no air bubbles above or below the tap and in the bore of the tap. Wipe any suspended liquid off the burette tip Zero the burette or record its initial volume. Re-wipe the burette tip. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 135 Laboratory Techniques for Environmental Technicians The indicator is added and you are ready to titrate An aliquot of the other solution is placed in the titration flask. (e.g. the analyte) Titration flask One solution is used to fill the burette (e.g. the standard) Apparatus used to perform titration Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 136 Laboratory Techniques for Environmental Technicians Titration Flask Rinse the flask with distilled water as the final step before adding the aliquot of solution. It may be left wet. Rinse a pipette with a small amount of the fill solution as the final step before filling. Obviously if there is any doubt about contamination, a more thorough cleaning will be required. Add the aliquot of solution. Sometimes an accurately weighed primary standard solid is used as the reactant, which needs a suitable amount of water added to dissolve it, prior to additions from the burette. Add a suitable indicator. Rough titration Add the solution from the burette in a slow steady stream to the titration flask while swirling vigorously. Observe the colour of the indicator carefully for some change in its shade or hue. This is a sign of an approaching end point. Stop the addition when you see the sudden colour change. Record this volume reading which is a rough end point. Obviously, with the first titration you will go many drops even many mL past the true end point but the purpose was to get a rough idea quickly rather than spend a lot of time adding single drops, mixing and waiting to see what happens. A 25 mL titration is about 500 drops and would be very slow and painful to do dropwise! Accurate titration Add the bulk of rough titration volume (say 80–95%) rapidly. Rinse the walls of the flask with distilled water to ensure all reactive species are in the mixing part of the flask This is essential when you are only few drops short of the end point. Complete the addition dropwise so that the end point is overshot by no more than one or two drops. Any more than this means you will need to reject this measurement. Repeat the titration procedure with fresh materials until you have an acceptable set of end point volumes (at least in triplicate with less than 0.2 mL range between all results). Calculate your mean, range, and absolute and relative precision. Titration results are only acceptable if they lie within a specified precision range (at least within 2% but 0.2% is possible with some standard methods). Some indicators fade on standing and so it will appear that your end point has changed but the general rule is to accept the first change which is permanent for 10–20 seconds. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 137 Laboratory Techniques for Environmental Technicians Ideal titration Ideally, the addition of a fraction of a drop (< 0.05 mL — the limit of reading of a burette), will produce a permanent colour change different from the previous colour. The volume added at this point is the true end point. (The equivalence point, which describes the volume added to provide the exact molar amounts of analyte and standard at the end point, can be used to calculate the ideal end point volume but it does not always match exactly what is observed in practice and you don’t usually know the answer anyway). Replicate determinations in an ideal analysis will all lie within 0.05 mL. The accumulated errors of the glassware used will produce a range of readings of at least this magnitude. All novices should aspire to performance which approaches these standards. Types of Standards used in Titration Standard solutions must have the following attributes: ◗ an accurately known concentration ◗ contain an active chemical species which reacts stoichiometrically with analytes of interest (i.e. you know the ratio of moles at the endpoint and the fact that the reaction is complete) ◗ reasonable shelf life in terms of maintaining a known concentration ◗ belong to a known chemical reaction class (e.g. acid or base, oxidant or reductant, chelating agent or complexible metal ion), so that correct matching of analyte and standard occurs. Two types of standard solutions are available in laboratories: primary standard solutions made up from primary standards by accurate weighing and dilution to volume in quantitative glassware. A primary standard is normally a solid chemical of precisely known purity (usually 99.9% or better) which is easy to weigh, tolerates exposure to the atmosphere and has a reasonable shelf life after opening the container. Naturally, it must have a reactant concentration which can be calculated from the original mass. Only very few chemicals qualify as primary standards, but examples exist for each of the major classes of reactions for which titration is used. Commonly, sodium carbonate solid is a primary standard alkali and potassium hydrogen phthalate solid is a primary standard acid. secondary standard solutions made up from secondary standards by approximate weighing and dilution to volume to give an approximate concentration. The secondary standard is defective in some way which prevents it being suitable for precise weighing. Generally it is because of unreliable purity, problems with exposure to the air or limited shelf life. Thus a solution containing the chemical species of interest can be made up, but with only an approximately known concentration. Hydrochloric, sulfuric, nitric and ethanoic (acetic) acids are all only suitable for use as secondary standard acids in their normal forms. Sodium Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 138 Laboratory Techniques for Environmental Technicians hydroxide, potassium hydroxide, barium hydroxide, and ammonia solution are only suitable for use as secondary standard alkalis in their normal forms. They must all then be standardised (e.g. titration with a primary standard) to establish the true concentration. Calculations Many methods exist to process titration data into analyte concentration and other information on composition. One method which restricts itself to moles and molarity is described here. All quantities which describe the analyte and the standard need to be converted to moles. All concentrations must be converted to molarity measured in moles per litre. Commonly used terms and their symbols and their units are given in the following table Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 139 Laboratory Techniques for Environmental Technicians Term Symbol Unit moles n mol molarity M mol. L–1 (mol/L) volume V mL but for some calculations it must be L mass m g formula weight FW g. mol–1 The formulae used to convert titration data to analyte data are found in below. Combination Formula moles of solute in n = M x V solution Equation No. Conditions 1 V must be in L moles of a solid n = m FW 2 molarity from moles M= n V 3 V must be in L molarity from mass M = (m FW) 4 V V must be in L Examples Moles and molarity calculations (a) How many moles of H+ in 25.0 mL of 0.1 M HCl ? data: M = 0.1 mol. L–1 and V = 25 mL which must be converted to L = 25 x 10–3 L Use Equation 1 to give n = 0.1 x 25 x 10–3 moles of HCl ANS. (b) n = 2.5 x 10-3 moles of Hcl and also of H+ How many moles of CO32– in 0.40 g of Na2CO3? data: FW = 23 + 23 + 12 + 16 + 16 + 16 = 106 g/mol m = 0.40 g Use Equation 2 to give n = 0.4 106 moles of Na2CO3 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 140 Laboratory Techniques for Environmental Technicians ANS. (c) n = 3.77 x 10–3 moles of Na2CO3 and also of CO32– What is the molarity of 25 mL of solution containing 2.5 x 10 –3 moles of Hcl ? data: V = 25 mL which must be converted to L = 25 x 10–3 L n = 2.5 x 10–3 moles Use Equation 3 to give 2.5 x 10–3 25 x 10–3 ANS. (d) M = 0.1 mol. L–1 of Hcl (or 0.1 M HCl) What is the molarity of 100 mL of solution containing 0.40 g of Na2CO3? data: V = 100 mL which must be converted to L = 100 x 10–3 L m = 0.40 g FW = 23 + 23 + 12 + 16 + 16 + 16 = 106 g/mol Use Equation 4 to give n = (0.4 106) (100 x 10–3) ANS. n = 0.0377 mol. L–1 of Na2CO3 (or 0.0377 M Na2CO3) Mole Ratios in Titration Reactions Not all titration reactions proceed in simple 1:1 ratios. The reaction ratio must be supplied, as in the case of standard methods, or checked by writing the balanced equation. Titration Calculations Calculate the number of moles in the volume of standard solution or in the mass of primary standard solid used. Determine the supplied values. reaction ratio from the balanced equation Calculate number of moles of the analyte which would number of moles of standard from step 1. Use the ratio from step 2. or use the react with the Use the volume of analyte solution to calculate its concentration. Examples Titration calculations 1. 25.0 mL of an unknown H2SO4 solution is titrated to an end point of 21.3 mL with 0.1034 M NaOH. Calculate its concentration. Step 1 Number of moles of NaOH = 0.1034 x 21.3 1000 = 2.20 x 10–3 mol. Step 2 H2SO4 + 2 NaOH Na2SO4 + 2H2O. Therefore, 1 mole of sulfuric acid reacts with 2 moles of NaOH, or 1 mole of NaOH is equivalent to 0.5 mole of sulfuric acid. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 141 Laboratory Techniques for Environmental Technicians or for every mole of NaOH consumed there was 0.5 mole of H2SO4 present. Step 3 Number of moles of H2SO4 = 0.5 x moles of NaOH = 1.10 x 10–3 mol. Step 4 M = 1.10 x 10–3 25 x 10–3 = 0.0440 mol. L–1. The concentration of H2SO4 was found to be 0.0440 mol. L–1 (0.0440 M H2SO4). 2. 19.4 mL of HCl solution is needed to obtain an end point with 0.153 g of Na 2CO3. Calculate the concentration of the HCl. Step 1 Number of moles of Na2CO3 = 0.153 106 = 1.44 x 10–3 mol. Step 2 2 HCl + Na2CO3 2 NaCl + H2O + CO2. Therefore, 2 moles of HCl react with 1 mole of Na2CO3, or 1 mole of HCl is equivalent to 0.5 moles of Na2CO3. or for every mole of Na2CO3 consumed there was 2 moles of HCl present. Step 3 Number of moles of HCl = 2 x moles of Na2CO3 = 2.88 x 10–3 mol. Step 4 M = 2.88 x 10–3 19.4 x 10–3 = 0.149 mol. L–1. The concentration of HCl was found to be 0.149 mol. L–1 (0.149 M HCl). Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 142 Laboratory Techniques for Environmental Technicians Practical: Practice titration Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ Results: 1. Observation of endpoint colour Colour of solution Colour of solution Colour of solution using screened using Using M.O. M.O. phenolphthalein Acid HCI Base NaOH End point 2. Practice Titration Concentration of supplied acid Volume of alkali pipetted into titration flask Volume of acid needed for titration end point 1 2 3 4 Average volume acid used for end point 3. Calculation of concentration of supplied alkali Concentration alkali = [Concentration acid x Volume acid] / Volume base = Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 143 Laboratory Techniques for Environmental Technicians Questions: What are the possible sources of error in the use of a burette? Why should all readings be written down and checked before draining or refilling burettes? Place each of the following pieces of glassware which may be used in a titration in the appropriate preparation column. Pipettes, burettes, volumetric flasks, titration conical flasks, beakers and bottles used to hold standard and other solutions. Used dry rinsed with solution to rinsed be placed in it water. with distilled Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 144 Laboratory Techniques for Environmental Technicians Practical: Ethanoic acid content of Vinegar Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine the ethanoic acid content of vinegar. Chemistry: Complete the equation to enable the mole ratio to be determined Sodium hydroxide + ethanoic acid sodium ethanoate + water NaOH(aq) + CH3COOH(aq) NaCH3COO(aq) + H2O(l) Results: Volume of original vinegar taken (mL) = Volume of diluted vinegar prepared (mL) = Vol. of diluted vinegar titrated (mL) = Concentration of NaOH used = Titration volumes Average titration value = Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 145 Laboratory Techniques for Environmental Technicians Calculations: moles of NaOH used = Concentration NaOH x Average titration / 1000 = moles ethanoic acid = moles of NaOH (because the mole ratio = 1:1) = Conc diluted ethanoic acid = moles ethanoic acid / (volume of aliquot/1000) = Conc original ethanoic acid = Concn of diluted vinegar x 10 (original vinegar solution) = Mass (g) ethanoic acid per L = Concn original ethanoic acid x 60 = % w/v ethanoic acid in vinegar = mass ethanoic acid per L / 10 = Questions: How would you titrate if brown vinegar had been used in place of white vinegar? How does your answer for the vinegar compared to food authority standards for legal vinegar? (The ethanoic acid content must be at least 4.0% w/v). Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 146 Laboratory Techniques for Environmental Technicians Practical: Sodium Carbonate Content of Washing Soda Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine the sodium carbonate content of washing soda by titration Equation: Sodium carbonate + hydrochloric acid Results: Mass of washing soda: (approx 3.6 g) = Size of volumetric flask: = Aliquot volume (mL) = Aliquot volume (L) = Concentration of HCl = Titration volumes (mL) Average titration (mL) Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 147 Laboratory Techniques for Environmental Technicians Calculations: moles of HCl = concn of HCl x average titration volume / 1000 = moles Na2CO3 = moles of HCl / 2 in aliquot = Concn Na2CO3 = moles Na2CO3(dil) aliquot volume(L) = Mass Na2CO3 in 250 mL = (Concn Na2CO3 4) x 106 volumetric flask = % sodium carbonate = mass sodium carbonate in 250 mL x100 Mass of initial washing soda = Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 148 Laboratory Techniques for Environmental Technicians Practical: Chloride by titration with silver nitrate Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine the salt content of potato chips Sodium is an essential element in the body – playing a major role in the functioning of nerves and the electrolyte balance in the blood. It also is an important substance in many other organisms and is partly responsible for the taste (or lack of it) in natural waters. When combined with chloride ions, sodium imparts a salty taste to water. Too high a concentration of these ions leads to the condition known as salinity, which destroys soil making it unsuitable for crop growing and general agricultural use. In drinking waters small amounts of sodium and chloride are actually desirable to help give the water taste. Water should be monitored to ensure that the levels remain acceptable for consumers. Chloride ions are relatively easy to monitor, as they may be analysed using a simple titration with silver nitrate. Procedure Weigh accurately about 3 g of chips (in triplicate) into 250 mL conical flasks Add about 50 mL of purified water and a small amount of calcium carbonate CaCO 3 powder and swirl for about 3 minutes Add about 1 mL of chromate indicator Titrate with standardised 0.1M silver nitrate until endpoint. Record your results and calculate the amount of salt in the potato chips Results 1 2 3 Mass of potato chips (g) Conc AgNO3 Volume AgNO3(mL) Calculations Balance the equation AgNO3 + NaCl AgCl(s) + NaNO3 Volume (L) Titrant = volume AgNO3 / 1000 Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 149 Laboratory Techniques for Environmental Technicians = Moles Ag+ = concentration AgNO3 x volume AgNO3 (L) = moles Ag+ = Moles chloride ion = Moles sodium chloride = moles chloride ion = Mass sodium chloride = moles chloride x FWt(NaCl) = % NaCl = mass sodium chloride / mass chips x 100 = Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 150 Laboratory Techniques for Environmental Technicians Practical: Determination of hardness in water Date Completed: ___________________ Teacher check _____________ Purpose Analyst signature __________ To determine the hardness of a supplied water sample. Water Hardness Water hardness is a common problem in outback Australia and areas whose water supply is derived from a river source. Hardness is defined as “the ability of water to cause precipitation of insoluble salts of higher fatty acids from soap solutions”. In other words water is hard when it won’t lather with soap! It is generally caused by high concentrations of Ca2+ and Mg2+ being present in the water. When used in water systems both of these hard ions (Ca2+ and Mg2+) play a role in forming coatings on the insides of pipes. In this practical you will determine the total hardness of a water sample using the EDTA titrimetric method. When reporting the result it is expected that the method used for determination will be quoted e.g. hardness (EDTA). Water hardness is removed chemically by the addition of sodium carbonate which causes the precipitation of Ca2+ and Mg2+ from solution. Another procedure (industrial use) utilises ion exchange resins called zeolites, which adsorb the divalent cations as they are pumped through them. This is important for reducing the level of boiler scale (precipitation caused by hard water) in pipes in industrial processing plants. Typical concentrations of ions (expressed as CaCO3 equivalents) – [from G. Laidler, Environmental Chemistry] Concentration Description of CaCO3 mg/L Water Quality 0-50 soft 50-100 moderately soft 100-200 slightly hard 200-300 hard > 300 very hard Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 151 Laboratory Techniques for Environmental Technicians Procedure Pipette a triplicate aliquot of the supplied water sample into 3 separate conical flasks. Add enough purified water to bring the volumes to about 50 mL. Add 5 mL of ammonium/ammonium chloride buffer and about 50 mg of Eriochrome black T indicator (your teacher will demonstrate the correct amount of powder) to each flask. Use the burette containing 0.01M EDTA solution, and titrate to the end point, which is the first permanent appearance of a blue colour. Note: the colour change is reasonably slow, so your first titration will probably overshoot. Repeat steps 2 - 3 until to you have three titration values within a 0.5 mL range. Record your best 3 titrations on the result sheet Complete the calculations to determine the hardness of your water sample. After your calculation compare your value with a table provided and report the relative hardness of your sample. Results Molarity of EDTA Titration Values M Titration 1 = mL Titration 2 = mL Titration 3 = mL Ave mL = Calculations A. Moles of CaCO3 = molarity EDTA x titration (mL) 1000 = B. Mass of CaCO3 (FWt 100) = A x 100 = Water Hardness (as CaCO3) = B x 1000000 mL of sample Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 152 Laboratory Techniques for Environmental Technicians = Water Hardness mg/L = mg/L Hardness Rating Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 153 Laboratory Techniques for Environmental Technicians Chapter 12: The Open Ended Problem Open ended project You have been working in your chemistry class and laboratory class towards an understanding of basic concepts in both the laboratory and chemistry, This task is designed to enable you to show your competence in both areas. You are to devise a simple laboratory project that will allow you to separate a mixture of sand, salt and sawdust and provide the percentage composition of the original mixture. You will be guided in class by your laboratory teacher and also you chemistry teacher. Requirements: Submission to your teacher of a flow chart showing the general laboratory procedure Identification of the physical And/or chemical properties you will use to make the separation A completed laboratory request form for each practical session Completed 5 minute risk assessments for each practical session A work journal indicating how your ideas changed each session A log book that records your data in a scientific manner A written report following the format provided by your teacher You will be required to submit a report at the conclusion of the project. The following questions could be useful as you design your project: What properties of each component could be useful for separation? What equipment will you require for the separation? Is it readily available in the laboratory? What hazards exist in your separation procedures? What SDS will you require? Do you need to know the original mass of the mixture? How many sessions do you need for the separation? Are there other methods that could work? Why did you choose a particular method? Why is it important to have a logbook for your data? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 154 Laboratory Techniques for Environmental Technicians Chapter 13: Appendix/Assessment information and completion record Assessment 1 For the basic Chemistry component there are 4 assignments to be completed Assessment 2 For the calculation section there are 2 assignments to be completed Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 155 Laboratory Techniques for Environmental Technicians Assessment 1 Assignment 1 a) Explain how you could separate the following mixtures in order to get the maximum yield of the highlighted component vegetable oil and water talcum powder and sugar iron filings and sand b) Give the symbol for the following elements Gold Helium Tungsten Sodium Nitrogen Calcium Iron Chlorine Molybdenum Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 156 Laboratory Techniques for Environmental Technicians Assignment 2 Complete the following chart Name Formula Name Formula potassium fluoride dinitrogen trioxide carbon disulfide phosphorus pentachloride calcium phosphate sodium carbonate silver nitrate lead (II) acetate ammonium sulfite aluminium carbonate hydrochloric acid sodium hydroxide sulfuric acid ammonium hydroxide hydrogen Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 157 Laboratory Techniques for Environmental Technicians Assignment 3 a) Using your Periodic Table, give an example for each of the following: a noble gas a metal with a valency of 1 a non-metal with a valency of 2 a liquid metal at room temperature a liquid non-metal at room temperature a non-metal which exists as diatomic molecules a transition metal a man-made metal a radioactive metal b) Find and record the atomic masses for the following elements iron ………………. iodine…………………… helium …………………… lead ………………. potassium ……………… sulfur …………………….. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 158 Laboratory Techniques for Environmental Technicians Assignment 4 a) Balance the following equations HCl(aq) + Fe(s) FeCl2(aq) + H2(g) (NH4)2Cr2O7(s) Cr2O3(s) + N2(g) + H2O(g) C4H10(g) + O2(g) CO2(g) + H2O(l) b) Describe how you would make up the following solutions for a senior chemistry class 500 mL of a 0.10 mol/L NaCl solution 500 mL of a 0.01 mol/L HCl solution from concentrated (10 M) HCl Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 159 Laboratory Techniques for Environmental Technicians Assessment 2 Assignment 1 PART A Given the following data and formulae, calculate the values of the unknown: (1 mark each) V 1. nRT P P = 1.22, R = 0.0821, T = 298, n = 0.0173 Answer V= _________________ n 2. m F F = 125, n = 2.45 x 10-3 Answer 3. m= _________________ E = 2 d (n + a)2 d = 39.4, n = 5.25 x 104, a = 1.67 x 10-3, is pi (see your calculator) Answer E= _________________ Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 160 Laboratory Techniques for Environmental Technicians 4. C= f [( V1 - V2) - (B1- B2)] p f = 0.1, p = 0.5, V1 = 9.7, V2 = 3.4, B1 = 1.2, B2 = 0.9 Answer C= _________________ Write 0.000234 in scientific notation Answer = _________________ Write 4.56 x 104 as a decimal number Answer = _________________ Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 161 Laboratory Techniques for Environmental Technicians PART B Perform the following conversions: (1 mark each) 7. 789 nm to m Answer 8. 4.25 x 10-4 kL to mL Answer 9. _________________ mL 270 mm2 to m2 Answer 10. _________________ m _________________ m2 62.3 kg to pounds (1 pound = 454g) Answer _________________ pounds Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 162 Laboratory Techniques for Environmental Technicians PART C Plot a calibration graph on the supplied graph paper, given the data below: (2 marks) Concentration (mg/L) Reading 0 0.01 2 0.18 4 0.35 6 0.55 8 0.74 Using your graph, determine the concentration of a sample with a reading of 0.44. (1 mark) Sample concentration Determine the concentration of a sample, given the data below for a calibration graph. (1 mark) slope 25.4 y-intercept 1.5 sample reading 89.4 Sample concentration Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 163 Laboratory Techniques for Environmental Technicians Assignment 2 PART A 1 How many moles are contained in 1.234 g of NaOH (FW = 40.0)? 2 What mass is 3.672 x 10-3 moles of lead chloride (2 marks each) (FW = 278.1)? 3 Calculate the molarity of a solution which has 3.84 x 10-4 moles in 25 mL of solution. 4 What molarity is produced when 1.329 g of NaCl is dissolved in 500 mL of solution? 5 What mass of NaCl (FW 58.44) would be required to make 111 mL of 0.111 M solution? 6 What is the concentration (in g/L) when 456 mg of KCl is dissolved in 1.5L of solution? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 164 Laboratory Techniques for Environmental Technicians 7 What mass of KNO3 would be required to prepare 200 mL of 100 mg/L? 8 What is the concentration (in %w/w, g/100g) of salt in soil if 0.0935 g of salt were found in 22.763 g of soil? 9 What mass of lead is present in 350 g of waste which has a lead concentration of 75 mg/kg? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 165 Laboratory Techniques for Environmental Technicians PART B (2 marks each) 1. What is the concentration of the diluted solution if 10 mL of 500 mg/L solution is diluted to 250 mL? 2. What volume of 4 g/L solution is required to make 2 L of 0.05 g/L solution? 3. What is the concentration of a solution if it was diluted by 25 mL to 100 mL and the diluted solution had a concentration of 3.56 mg/L? 4. To what volume should 10 mL of 0.05 M solution be diluted, so that the diluted solution has a concentration of 0.0025 M? 5. How many moles are present in 250 mL of solution if a 20 mL aliquot of it contained 2.75 x 10-3 moles? Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 166 Laboratory Techniques for Environmental Technicians Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 1