X-gal staining on frozen sections
advertisement
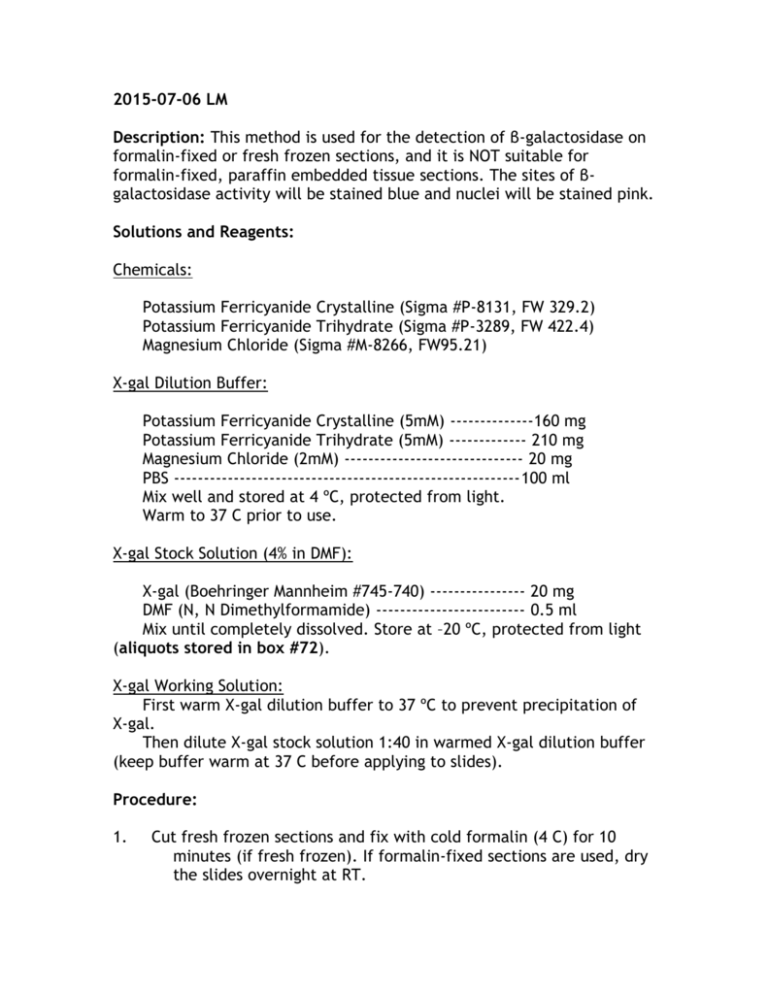
2015-07-06 LM Description: This method is used for the detection of β-galactosidase on formalin-fixed or fresh frozen sections, and it is NOT suitable for formalin-fixed, paraffin embedded tissue sections. The sites of βgalactosidase activity will be stained blue and nuclei will be stained pink. Solutions and Reagents: Chemicals: Potassium Ferricyanide Crystalline (Sigma #P-8131, FW 329.2) Potassium Ferricyanide Trihydrate (Sigma #P-3289, FW 422.4) Magnesium Chloride (Sigma #M-8266, FW95.21) X-gal Dilution Buffer: Potassium Ferricyanide Crystalline (5mM) --------------160 mg Potassium Ferricyanide Trihydrate (5mM) ------------- 210 mg Magnesium Chloride (2mM) ------------------------------ 20 mg PBS ----------------------------------------------------------100 ml Mix well and stored at 4 ºC, protected from light. Warm to 37 C prior to use. X-gal Stock Solution (4% in DMF): X-gal (Boehringer Mannheim #745-740) ---------------- 20 mg DMF (N, N Dimethylformamide) ------------------------- 0.5 ml Mix until completely dissolved. Store at –20 ºC, protected from light (aliquots stored in box #72). X-gal Working Solution: First warm X-gal dilution buffer to 37 ºC to prevent precipitation of X-gal. Then dilute X-gal stock solution 1:40 in warmed X-gal dilution buffer (keep buffer warm at 37 C before applying to slides). Procedure: 1. Cut fresh frozen sections and fix with cold formalin (4 C) for 10 minutes (if fresh frozen). If formalin-fixed sections are used, dry the slides overnight at RT. 2. 3. 4. 5. 6. 7. 8. Wash slides with 3 changes of PBS for 5 minutes each and then rinse in distilled water. Incubate slides in X-gal working solution at 37 C for 24 hours (use humidified chamber to prevent slides from drying). Rinse sections in PBS for 2x5 minutes Rinse with distilled water briefly. Counterstain with nuclear fast red for 3-5 minutes. Rinse in distilled water. Mount DIRECTLY with aqueous mounting medium.