Supplementary material Tissue-specific response of neonatal rats to
advertisement

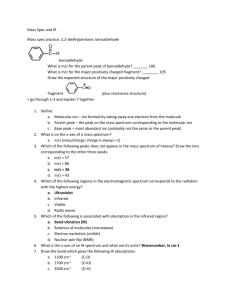
Supplementary material Tissue-specific response of neonatal rats to selenite-induced oxidative stress and to concurrent administration of highbush blueberry leaf polyphenols. Anastasia-Varvara Ferlemi1, Olga E. Makri2, Penelope G. Mermigki1, Dimitrios Anagnostopoulos4, Nikolaos S. Koulakiotis1,4, Marigoula Margarity3, Anthony Tsarbopoulos4,5, Constantinos D. Georgakopoulos2, Fotini N. Lamari1* 1 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece 2 Department of Ophthalmology, Medical School, University of Patras, 26504 Patras, Greece 3 Laboratory of Human & Animal Physiology, Department of Biology, University of Patras, 26504 Patras, Greece 4 The Goulandris Natural History Museum, GAIA Research Center, Bioanalytical Department, 14562 Kifissia, Greece 5 Department of Pharmacology, University of Athens Medical School, 11527 Athens, Greece *Corresponding author: Dr. Fotini N. Lamari Materials and methods HPLC-DAD Analysis Instrumentation Results Characterization of polyphenols in blueberry leaf extract In total, 20 different compounds were found in Crude leaf extract and three fractions (table 1); analytical description of the phytochemicals that were detected is presented hereinafter. Phenolic acids Peaks 1, 3 and 4 were phenolic acids belonging to the subclass of hydroxycinnamic acids. Quinic and caffeic acid were unambiguously identified, along with the product of their esterification (caffeoyl quinic acid or chlorogenic acid), which is the major component. Caffeic acid showed a fragment ion at m/z 135 corresponding to the loss of COO- unit (44 mau). Peaks 3i and 3ii could be identified as two of the three main possible isomers. Both of them yielded a main fragment ion at m/z 191 corresponding quinic acid, due to a loss of caffeoyl moiety. Peak 3i was identified as 3caffeoylquinic acid using an external standard. Nevertheless, we could not be certain if the compound eluting at 2.21 min (peak 3ii) was 4- caffeoylquinic acid or 5caffeoylquinic acid. Flavonols Thirteen different flavonol glycosides were detected and four of them are derivatives of myricetin: myricetin-3-O –rutinoside (peak 6), myricetin-3-O- glucoside (peak 7i) and myricetin-3-O-galactoside (peak 7ii). A compound eluting at 3.08 (peak 8) could be assigned as myricetin-3-O-arabinoside or xyloside, but we did not have an authentic standard to confirm its identity. All peaks had fragmentation at m/z 316, something that indicated the presence of aglycon myricetin. Fragmentation of the molecular ion of peak 6 by increasing collision energy resulted in the formation of [Μ-2Η-308]- ion at m/z 316 and demonstrated the loss of deprotonated rutinoside. Taking into account the chromatographic behavior of peak 7 (double peak), it was considered that samples contain both glycosides of myricetin. Peak 8 was characterized as a myricetin bonded with a pentose moiety due to the formation of characteristic ion at m/z 316 after the loss of 132 amu. The neutral losses of 132, 162, 146 and 152 mass units allowed the identification of pentosides (arabinose or xylose), hexosides (glucose or galactose) and deoxyhexoside (rhamnose) respectively. Seven derivatives of quercetin were detected: quercetin-3-rutinoside (peak 9), quercetin-3-glucoside (peak 10i), quercetin-3-galactoside (peak 10ii), quercetin-3arabinoside (peak 12i), quercetin-3-xyloside (peak 12ii), quercetin-3-rhamnoside (peak 13), quercetin-3- (6΄΄-acetyl)-glucoside (peak 15). Study by MS analysis revealed that fragmentation of these compounds produced an overwhelming major ion at m/z 300 (rather than at m/z 301 ([quercetin-H]-) by losing sugar moiety and forming of the quinone radical ion. So in the mass spectrum, for each of peaks 9, 10i10ii, 12i-12ii, 13 and 15 were presented the following fragment ions: [M-2H-308]-, [M-2H-162]-, [M-2H-132]-, [M-2H-146]- and [Μ-2H-162-42]- respectively. Regarding peak 15, the loss of (162+42)=204 mau indicated that the compound containing an acetyl group (42 mau) attached to an hexose. Peaks 10i-10ii and 12i12ii showed one more fragment ion at m/z 271 corresponding to the loss of -(H+CO) unit (30 mau) from quercetin. Also, peak 16 was characterized as quercetin and was detected in the blueberry leaf extract (Crude) and AcOEt fraction. However, for more detailed identification of the sugar units of quercetin glycosides, the retention times were compared with those of authentic standards used for identification of quercetin3-O –rutinoside, glucoside and galactoside with HPLC-DAD analysis. Furthermore, a derivative of kaempferol was identified, kaempferol-3-O-rutinoside (peak 11), which had a [M-H]- at m/z 593 and a fragment [M-H-308]- at m/z 285. This fragment is typical for a deprotonated kaempferol after the loss of a rutinoside. Proanthocyanidins Peak 2 eluting at 1.85 min was only identified in AcOEt fraction and was characterized as prodyanidin B1 or B2. The two molecules have common fragments, thus the accurate determination of this compound became difficult. It was confirmed by a fragment ion at m/z 289 indicated the loss of the one of the two monomers. The fragment at m/z 425 was derived from Retro-Diels-Alder rearrangement of the heterocyclic ring. Sequential loss of water yield a fragment at m/z 407. In addition to dimer procyanidin, phenylpropanoid substituted catechins (common name: cinchonains) were detected as well as dimers consisting of catechin and cinchonain. Peaks 5 and 14 detected in blueberry leaf extract and AcOEt fraction were determined as kandelin (A1 or A2) and cinchonain (Ia or Ib). Taking into account the mass accuracy and literature data, they may belong to cinchonain Ia or Ib but the complete identification was not feasible.