South Pasadena • Chemistry Name 4 • Salts and Solutions Period
advertisement

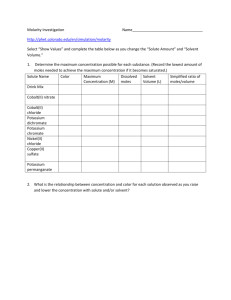
South Pasadena • Chemistry Name 4 • Salts and Solutions Period Date SOLUTIONS SIMULATIONS Run these simulations and complete these questions using complete sentences Concentration Simulation Run the Concentration Simulation at: http://phet.colorado.edu/en/simulation/concentration 1. Write the color of each solution. (a) Drink mix (e) Potassium chromate (b) Cobalt (II) nitrate (f) Nickel (II) chloride (c) Cobalt chloride (g) Copper sulfate (d) Potassium dichromate (h) Potassium permanganate 2. State a claim about the relationship between the color of the solution and its concentration. Provide evidence supporting this claim. Claim: Evidence: 3. What are two ways to increase the concentration of the solution? Based on these two ways, what factors and in what way do these factors affect the concentration? (e.g. “One factor that determines the solution’s concentration is the . As the increases, the concentration .”) 4. What is one way to decrease the concentration of the solution? 5. What happens to the concentration when the solution drained? Why? 6. Watch what happens when lots of each solute is added to the solution and then evaporated. Explain what happens to the solute and the concentration of the solution as more solute is added. 7. Find the concentration of the solution for each solute when it is saturated. (a) Drink mix (e) Potassium chromate (b) Cobalt (II) nitrate (f) Nickel (II) chloride (c) Cobalt chloride (g) Copper sulfate (d) Potassium dichromate (h) Potassium permanganate 8. List the solutes from the least soluble to the most soluble. Molarity Simulation Run the Molarity Simulation at: http://phet.colorado.edu/en/simulation/molarity This simulation is similar to the Concentration Simulation. Click on “Show values.” 1. State the relationship between the amount of solute and the solution concentration while the solution volume remains constant. The amount of solute is [directly | inversely] related to the solution concentration because when you double the amount of solute while keeping the volume constant, the solution concentration is . 2. State the relationship between the solution volume and the solution concentration while the amount of solute remains constant. (See example in question 1.) 3. State the relationship between the amount of solute and the solution volume while keeping the solution concentration constant. (Hint: When you double the amount of solute, what do you have to do to the solution volume to keep the same concentration?) 4. Find the concentration of each solution when it is saturated. (a) Drink mix (f) (b) Cobalt (II) nitrate (g) (c) Cobalt chloride (h) (d) Potassium dichromate (i) (e) Gold (III) chloride Potassium chromate Nickel (II) chloride Copper sulfate Potassium permanganate 5. Can a solution be more than saturated? What happens to the solution and its concentration as you continue to increase amount of solute beyond saturated? Sugar and Salt Solutions Simulation Run the Sugar and Salt Solutions Simulation at: http://phet.colorado.edu/en/simulation/sugar-and-salt-solutions Run the “Macro” tab. 1. Place the conductivity probe into the solution. The brightness of the light bulb shows how well the solution conducts electricity. State the relationship between the amount of solute and the solution’s conductivity. (a) As more salt is added to the solution, the solution’s conductivity . (b) As more sugar is added to the solution, the solution’s conductivity . Observe how adding water, evaporating the solution, and draining the solution affects the conductivity of the solution. Run the “Micro” tab. 2. What types of compound are salt (sodium chloride) and sugar (sucrose) (i.e. ionic or covalent)? How do you know? (a) Salt (sodium chloride) is a compound because . (b) Sugar (sucrose) is a compound because . 3. How do these two solutes dissolve differently in the solution? 4. Suggest a reason for why salt and sugar conduct electricity differently. Run the “Water” tab. 5. Click on “Show water partial charges”. Describe the water molecule, including: what the molecule looks like, how the charges are arranged in the molecule, and how the water molecules move in the sample. 6. Place the salt in solution. What happens? 7. Click “Pause.” Observe how the δ+ end of water molecule tends to surround the Cl− ion, and the δ− end of the water molecule tends to surround the Na+ ion. Why does this happen? 8. Place the sugar molecule in water. (It is more distinguishable if the “Show Sugar highlight” box is checked.) What happens? 9. Click on “Sugar in 3D.” Compare the space-filling model of the compound with the ball-and-stick model.