SOP BAL-001 - Biotech Virtual Mentors
advertisement
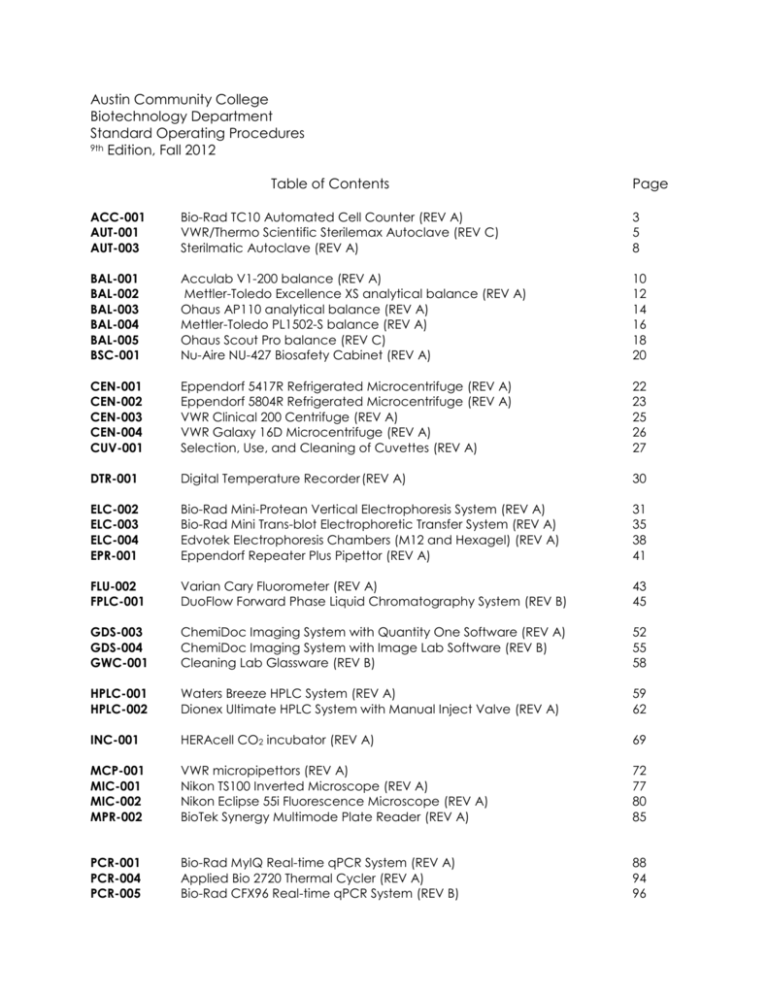
Austin Community College Biotechnology Department Standard Operating Procedures 9th Edition, Fall 2012 Table of Contents Page ACC-001 AUT-001 AUT-003 Bio-Rad TC10 Automated Cell Counter (REV A) VWR/Thermo Scientific Sterilemax Autoclave (REV C) Sterilmatic Autoclave (REV A) 3 5 8 BAL-001 BAL-002 BAL-003 BAL-004 BAL-005 BSC-001 Acculab V1-200 balance (REV A) Mettler-Toledo Excellence XS analytical balance (REV A) Ohaus AP110 analytical balance (REV A) Mettler-Toledo PL1502-S balance (REV A) Ohaus Scout Pro balance (REV C) Nu-Aire NU-427 Biosafety Cabinet (REV A) 10 12 14 16 18 20 CEN-001 CEN-002 CEN-003 CEN-004 CUV-001 Eppendorf 5417R Refrigerated Microcentrifuge (REV A) Eppendorf 5804R Refrigerated Microcentrifuge (REV A) VWR Clinical 200 Centrifuge (REV A) VWR Galaxy 16D Microcentrifuge (REV A) Selection, Use, and Cleaning of Cuvettes (REV A) 22 23 25 26 27 DTR-001 Digital Temperature Recorder (REV A) 30 ELC-002 ELC-003 ELC-004 EPR-001 Bio-Rad Mini-Protean Vertical Electrophoresis System (REV A) Bio-Rad Mini Trans-blot Electrophoretic Transfer System (REV A) Edvotek Electrophoresis Chambers (M12 and Hexagel) (REV A) Eppendorf Repeater Plus Pipettor (REV A) 31 35 38 41 FLU-002 FPLC-001 Varian Cary Fluorometer (REV A) DuoFlow Forward Phase Liquid Chromatography System (REV B) 43 45 GDS-003 GDS-004 GWC-001 ChemiDoc Imaging System with Quantity One Software (REV A) ChemiDoc Imaging System with Image Lab Software (REV B) Cleaning Lab Glassware (REV B) 52 55 58 HPLC-001 HPLC-002 Waters Breeze HPLC System (REV A) Dionex Ultimate HPLC System with Manual Inject Valve (REV A) 59 62 INC-001 HERAcell CO2 incubator (REV A) 69 MCP-001 MIC-001 MIC-002 MPR-002 VWR micropipettors (REV A) Nikon TS100 Inverted Microscope (REV A) Nikon Eclipse 55i Fluorescence Microscope (REV A) BioTek Synergy Multimode Plate Reader (REV A) 72 77 80 85 PCR-001 PCR-004 PCR-005 Bio-Rad MyIQ Real-time qPCR System (REV A) Applied Bio 2720 Thermal Cycler (REV A) Bio-Rad CFX96 Real-time qPCR System (REV B) 88 94 96 2 PHM-002 Accumet AB15 pH meter (REV A) 102 RCV-001 Receiving and Storing Materials (REV A) 105 SOL-001 SPC-001 SPC-002 SPC-003 SPC-004 SPC-008 Labeling solutions (REV A) Genesys 10 Spectrophotometer (REV A) BioMate 3 Spectrophotometer (REV A) Nanodrop 1000 Spectrophotometer (REV A) Perkin-Elmer Lambda Bio+ Spectrophotometer (REV A) Nanodrop 2000 Spectrophotometer (REV A) 107 110 114 118 119 122 TAC-001 Tachometer (REV A) 124 VAC-001 Integra VacuSafe Aspirator (REV A) 126 WPS-001 RODI Water Purification System (REV A) 127 Record of Revisions to Standard Operating Procedures 129 3 SOP ACC-001 TC10 AUTOMATED CELL COUNTER Revision A, June 7, 2011 Title: Bio-Rad TC10 Automated Cell Counter 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for use and maintenance of the Bio-Rad TC10 Automated Cell Counter. 2.0 SUMMARY OF METHOD MATERIALS 1. 2. 3. 4. 5. TC10 Automated Cell Counter TC10 Sample slide Trypan blue stain Cell sample P20 micropipettor and tips PART A: BASIC ABSORBANCE, TRANSMITTANCE, AND CONCENTRATION READINGS AVOID touching the optical surface of the slides. 1. Turn on the TC10 Automated Cell Counter via the green square button on side bottom of equipment. 2. Wait for the instrument to initialize. 3. Prepare slide to be analyzed while waiting. 4. Select options on the home screen by pressing the [UP] or [DOWN] key on the navigation key pad. 5. When your selection is highlighted in green, press [ENTER] on the navigation key pad. 6. Choose Count Cells and insert the prepared sample slide into the slide port of the cell counter. The counter will focus and count cells. 7. The Current Count screen will appear and display the total count of your sample, the date and time, and the option to calculate the dilution of your sample. In this screen, you will also be able to view an image of your sample, print data, and view a histogram. To toggle between these options press the [UP] or [DOWN] key and press [ENTER]. Loading slide using trypan blue stain Combine 10 µl cell suspension with 10 µl of trypan blue dye in a microcentrifuge tube. Pipet gently up and down to mix. Pipet 10 µl of the mixture into a TC10 sample slide. Place pipet tip at 45 degree angle at the bottom of sample loading area (the half-circle at outer end of the chamber). Only load into the outer end of the counting slide. The center notches are for distribution purposes. 4 8. Any time you want to return to the Home screen press the [HOME] key on the navigation key pad. The cell counter demonstrates high reproducibility within the range of 5X104 to 1X107 cells/mL. 9. The TC10 Automated cell counter allows you to print if connected to a printer. Select “Previous Counts” using the [UP] or [DOWN] key press [ENTER] and press [ENTER] again to print. Throw away the slide after they are used. These slides may not be reused. 10. You may save counts and images to a USB storage device. Insert a device into the USB port. Select Export Previous Counts on the Home screen and press [ENTER] to send to the device. 11. Use the Dilution Calculator to find the concentration of your sample. 5 SOP AUT-001 OPERATION OF VWR/THERMO SCIENTIFIC STERILEMAX AUTOCLAVE Revision C, August 10, 2012 Title: Operation of VWR/Thermo Scientific Sterilemax Autoclave 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the Sterilemax autoclave. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS 1. Sterilemax autoclave 2. 4-7 L deionized water 3. cleaning solution and scouring pad PART A: OPERATION 1. Plug autoclave into a 115-V outlet. Turn on autoclave using switch on bottom right edge of front of machine. 2. Check water level by opening reservoir on top right side. Water should be at least six inches from bottom of reservoir and should not be discolored or cloudy. This autoclave automatically cycles water back into the reservoir, so the water is often contaminated. Water should be drained and replaced at least once a week. 3. If water appears contaminated, empty the reservoir by following instructions in Part B. If water level is too low, add tap water until water is at least 6 inches high. Replace lid on reservoir. 4. Open chamber door by turning handle counter-clockwise until handle swings outward. Squeeze the door release panel on bottom left edge of door to release the door fully. Pull forward to open. 5. Load items to be autoclaved into chamber. There are three racks which are all removable, but never operate the autoclave without the lowest rack or glassware could break. 6. Close the chamber door and swing the handle to the right until its axis is snugly This autoclave will accommodate three 2-L flasks, but nothing taller than that. Be sure to mark items with a small piece of autoclave tape and check for darkened stripes to indicate sterilization. 6 placed in the door. Turn the handle clockwise until it is very difficult to turn further. The packs cycle is designed for lab packs, which are containers full of biohazardous sharps and Plaster of Paris. You will not need to use this cycle. 7. Press “start” to choose a program. Each program is suited to sterilize a particular material. 8. If you are sterilizing liquids, choose the liquid cycle. This will heat to a higher temperature than the other cycles and exhaust more slowly to prevent evaporation and splashing. Be certain that all caps have been loosened or containers can explode under high pressure! 9. For wrapped materials (such as sterilizing pouches full of instruments or for biohazard-contaminated materials in a biohazard bag), choose the wrapped cycle. This will exhaust quickly. 10. For unwrapped materials (such as a fermentation vessel or other empty glassware), choose the unwrapped cycle. This will exhaust very quickly. 11. Once you have pressed the button corresponding to the program you want, the parameters of the program will appear. You can change these if you wish, but in most cases you will want to leave the default parameters. 12. Press “start” and the program will begin. 13. When the sterilization is complete, the machine will beep and display a command to open the chamber door to allow the chamber to exhaust. 14. Allow the drying cycle to complete before you attempt to remove items, especially hot liquids. 15. When drying is complete, open door completely and stay to the right to avoid steam burns. 16. Remove trays or items using insulated Open chamber as described in step 4, but stay to the right side and keep your hand and arm away from the opening so you are not burned by steam escaping. 7 gloves or hot hands to avoid burns. Place on a lab bench or cart to cool to a reasonable temperature before attempting to use. PART B: MAINTENANCE 1. At least once weekly, the reservoir should be drained completely of water and refilled. To drain, open the chamber door when cool. Locate the drain hose and find the working end (has a white plastic gasket). 2. Place the non-working end into the sink. Insert the working end of the drain hose into the drain plug, located inside the door frame on the lower right. 3. Reservoir will begin draining immediately. Drain completely and remove hose by pressing on release button (just below the drain plug). 4. Refill reservoir with 4-7 liters of deionized water. 5. At least once monthly, the autoclave chamber should be scrubbed using a plastic scouring pad and a special cleaner (found on top of the machine). PART C: CHECKLIST □ Check for adequate water □ Loosen caps on bottles and DO NOT OVERFILL (more than 1/3 full) □ Load chamber, close and start cycle □ Remove your items when finished 8 SOP AUT-003 USE AND MAINTENANCE OF STERILMATIC AUTOCLAVE Revision A, February 22, 2011 Title: Use and Maintenance of Sterilmatic Autoclave 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for using the Sterilmatic autoclave. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS 1. Sterilmatic autoclave 2. 4 L tap water PART A: OPERATION 1. Load items into chamber. Do not crowd the items too closely, or the steam will not penetrate between them. 2. Make sure chamber drain is in closed position. Pour 4 L of tap water into the chamber until it reaches the fill line. Do not exceed the fill line! 3. Grasp the door handle and hold it in a vertical position while pulling down until bottom of door rests in the door opening. Make sure the rubber seal is flush with the door. Then push door handle down to engage the lock. 4. Select the correct exhaust type. “Liquids” is to be used for any liquids, while “Instruments” can be used for all other items. 5. Set the temperature to 121ºC. 6. If you are sterilizing liquids, choose the liquid cycle. This will heat to a higher temperature than the other cycles and exhaust more slowly to prevent evaporation and splashing. Be certain that all caps have been loosened or containers can explode under high pressure! Mark items with indicator tape. Make sure that closed vessels, especially glass bottles, are vented (lid not entirely closed). 9 7. Turn the timer to start operation. Timer should always be set to at least 15 minutes, as this is the minimum time for sterilization to be effective. 8. When the chamber reaches the selected temperature, the timer will begin. 9. When cycle is complete, allow the chamber pressure to drop to zero before attempting to open the door. 10. Open door completely by pulling up on the door handle. Stay to the side to avoid steam burns. 11. Remove trays or items using insulated gloves or hot hands to avoid burns. Place on a lab bench or cart to cool to a reasonable temperature before attempting to use. PART B: MAINTENANCE At the end of each day, the chamber should be drained completely. Place a bucket under the drain and open drain valve. Close valve when draining is complete and dispose of water (do not reuse). PART C: CHECKLIST □ Close drain valve and add 4 L tap water to the chamber □ Loosen caps on bottles and DO NOT OVERFILL (more than 1/3 full) □ Load chamber and close door □ Choose appropriate cycle □ Start timer □ Remove your items when finished □ Open the drain valve to drain the chamber 10 SOP BAL-001 OPERATION AND CALIBRATION OF ACCULAB MODEL VI-200 ELECTRONIC BALANCE Revision A, February 22, 2011 Title: Operation and calibration of Acculab V1-200 Balance 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for the proper operation and calibration of the Acculab Model VI-200 Balance. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS 1. VI-200 balance 2. standard 200 g mass PROCEDURE PART A: CALIBRATION 1. Turn on balance and allow it to warm up for at least 30 minutes. Use only a 12V AC adapter with a negative tip to supply power. Unplug when not in use. 2. Remove all items from the weighing pan and tare the balance by pressing the TARE key. 3. Check that the balance is in gram mode, indicated by a stable arrow on the display next to the g symbol on the casing. If the balance is not in gram mode, press and release the CAL/MODE key until the arrow is next to g. 4. After observing a stable zero reading (0.00) press and hold the CAL/MODE key. After four seconds the unit will beep and the calibration weight (+200) will appear on the display. Release the CAL/MODE key. 5. Gently place the 200 gram standard mass on the weighing pan. 6. The + sign will disappear from the display. Wait seven to ten seconds and the unit will beep. 7. The displayed weight (200) will disappear and then reappear as an active value. Calibration is now complete. Do not disturb the balance during calibration, and avoid vibrations and air currents. If the calibration weight value remains on the display, an improper weight has been used. Select the proper weight (200g) and repeat the calibration. 11 PART B: OPERATION 1. Remove all items from the weighing pan and tare the balance by pressing the TARE key. 2. Check that the balance is in gram mode, indicated by a stable arrow on the display next to the g symbol on the casing. 3. Place a weigh boat, weigh paper, or small vessel (such as an Erlenmeyer flask) on the balance pan. 4. Press the TARE key to tare the weight of the weighing vessel. 5. Place the substance to be weighed into the weighing vessel and allow the value to stabilize on the display. 6. Record the mass of the substance, remove the weighing vessel and contents from the balance and turn off. 7. Check that the entire balance and the area around it are clean and dry. Reagents can be weighed directly into any vessel as long as it does not weigh more than 200 grams (the maximum capacity of this model). If weighing a chemical, use a clean, dry spatula to transfer it into the vessel, or if a free-flowing solid, gently sift it from the container. Record any error messages or drifting mass values in the comments section of the log. If the balance was dirty or wet or the balance area was messy when you began working, record that in the comments section. 12 SOP BAL-002 OPERATION AND CALIBRATION OF METTLER-TOLEDO EXCELLENCE XS MODEL ANALYTICAL BALANCE Revision A, May 14, 2010 Title: Operation and calibration of M-T Excellence analytical balance 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for the proper operation, calibration and maintenance of the M-T Excellence analytical balance. 2.0 SUMMARY OF METHOD MATERIALS 1. VI-200 balance 2. standard 200 g mass PROCEDURE PART A: CALIBRATION The Excellence XS analytical balance has fully automatic calibration functions, so it needs to be calibrated by hand very rarely. COMMENTS Use of this SOP indicates that the user has read the M-T Excellence XS user manual. The manual must be read prior to use of this SOP. PART B: OPERATION 1. CAUTION: Do not overload balance. Maximum capacity is 220 g. 2. Place a weigh boat or paper on the weighing pan, close all draft shields, and tare the balance by pressing the TARE key. “Net” will appear in the display to indicate that all displayed weights will be net values. 3. Open the draft shield and insert the items to be weighed. Never sift chemicals from a container directly into the analytical balance. Spillage could devastate the instrument. Instead, carefully transfer material using a spatula. 4. Close the draft shield and wait for the stability detector icon, a small circle, disappears from the display. The presence of this icon indicates that the balance is NOT stable and you should wait for it to disappear. Do not use the ZERO key for taring. This is only used for initial setup of the balance. Do not disturb the balance during operation, and avoid vibrations and air currents. If weighing a chemical, use a clean, dry spatula to transfer it into the vessel. Record any error messages or drifting mass values in the comments section of the balance log, found in the equipment log book. If the balance was dirty or wet or the balance area was messy when you began working, record that in the comments section. 13 5. Record the mass and remove all items from the weighing pan. Turn off balance by pressing the ON/OFF key and holding down until balance is off. 6. Check that the entire balance and the area around it are clean and dry. Remove any material from the inside of the balance by gently sweeping it out with a brush. Avoid pressing down on the balance pan while cleaning. Do not use water to clean. Wipe any material away from the area around the balance. Clean spatulas or other weighing tools thoroughly with soapy water and dry completely before replacing. Never replace a used spatula to its storage container before cleaning it thoroughly. 14 SOP BAL-003 OPERATION AND CALIBRATION OF OHAUS AP110 ANALYTICAL BALANCE Revision A, September 11, 2007 Title: Operation and calibration of Ohaus AP110 analytical balance 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for the proper operation, calibration and maintenance of the Ohaus analytical balance. 2.0 SUMMARY OF METHOD MATERIALS 1. Ohaus AP110 balance 2. standard 100 g mass PROCEDURE PART A: CALIBRATION 1. Make sure there is no load on the balance pan and close the draft shield. 2. Hold down the ON/TARE button until “MENU” appears. 3. Press the ON/TARE button once and “AUTO” will appear. 4. Press the MODE button twice until “User” appears. 5. Press the ON/TARE button and “100.0000” should appear. This is the value used to last calibrate the balance. Press ON/TARE repeatedly to confirm each digit of this number is correct, then press again and “0 g” should appear. 6. Press ON/TARE once more to start zero mass calibration. “-C-“ should appear and the balance will initiate calibration. 7. When “CAL 100g” appears, place a 100-g mass on the balance pan. Press ON/TARE to initiate 100 g calibration. 8. “200.0000” should appear. Calibration is complete. COMMENTS Use of this SOP indicates that the user has read the Ohaus AP110 user manual. The manual must be read prior to use of this SOP. 15 PART B: OPERATION CAUTION: Do not overload balance. Maximum capacity is 100 g. 1. Turn balance on by pressing the ON/TARE button. 2. Place a weigh boat or paper on the weighing pan, close all draft shields, and tare the balance by pressing the TARE key. 3. Open the draft shield and insert the items to be weighed. Never sift chemicals from a container directly into the analytical balance. Spillage could devastate the instrument. Instead, carefully transfer material using a spatula. 4. Close the draft shield and wait for the stability indicator, a small “s”, to appear on the left of the display. If weighing a chemical, use a clean, dry spatula to transfer it into the vessel. Record any error messages or drifting mass values in the comments section of the balance log, found in the equipment log book. If the balance was dirty or wet or the balance area was messy when you began working, record that in the comments section. 5. Record the mass and remove all items from the weighing pan. 6. Turn off balance by pressing the ON/OFF key and holding down until balance is off. 7. Check that the entire balance and the area around it are clean and dry. Remove any material from the inside of the balance by gently sweeping it out with a brush. Wipe any material away from the area around the balance. Avoid pressing down on the balance pan while cleaning. Do not use water to clean. 8. Clean spatulas or other weighing tools thoroughly with soapy water and dry completely before replacing. Never replace a used spatula to its storage container before cleaning it thoroughly. 16 SOP BAL-004 OPERATION AND CALIBRATION OF METTLER-TOLEDO PL1502-S ELECTRONIC BALANCE Revision A, February 22, 2011 Title: Operation and calibration of Mettler-Toledo PL1502-S Balance 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for the proper operation and calibration of the Mettler-Toledo PL1502-S Balance. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS 1. PL1502-S balance 2. standard 200 g mass PROCEDURE PART A: CALIBRATION 1. Turn on balance and allow it to warm up for at least 30 minutes. Do not disturb the balance during calibration, and avoid vibrations and air currents. 2. Remove all items from the weighing pan and tare the balance by pressing the TARE key. 3. Press the CAL key to start calibration. 4. The maximum weight value, 200.000 grams, will flash on the LCD. 5. Gently place the 200 gram standard mass on the weighing pan. 6. When 0.00 g flashes on the LCD, remove the 200 g weight. 7. The adjusting is finished when the message CAL done appears on the display, followed by 0.00 g. PART B: OPERATION 1. Place a weigh boat, weigh paper, or small vessel (such as an Erlenmeyer flask) on the balance pan. 2. Press the 0/T key to tare the weight of Reagents can be weighed directly into any vessel as long as it does not weigh more than 200 grams (the maximum capacity of this model). If weighing a chemical, use a clean, dry 17 the weighing vessel. 3. Place the substance to be weighed into the weighing vessel and allow the value to stabilize on the display. The stability detector “º” will disappear when the reading is stable. 4. Record the mass of the substance, remove the weighing vessel and contents from the balance and turn off. 5. Check that the entire balance and the area around it are clean and dry. spatula to transfer it into the vessel, or if a free-flowing solid, gently sift it from the container. 18 SOP BAL-005 OPERATION AND CALIBRATION OF OHAUS SCOUT PRO ELECTRONIC BALANCE Revision C, June 6, 2012 Title: Operation and calibration of Ohaus Scout Pro SP402 Balance 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for the proper operation and calibration of the Ohaus Scout Pro Balance. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS 1. Scout Pro balance 2. standard 200 g mass PROCEDURE PART A: CALIBRATION 1. Remove all items from the weighing pan. 2. Do not disturb the balance during calibration, and avoid vibrations and air currents. Press and hold the ON/ZERO key until “MENU” appears on the display. 3. After you release the ON/ZERO key, “CAL” will display on the screen. 4. Press the ON/ZERO key again to start calibration. 5. “C” will appear on the display and the balance will calibrate to mass = 0 g. Do not place anything on the balance at this time. 6. Press the ON/ZERO key to display “C 200” (flashing). 7. Gently place the 200 gram standard mass on the weighing pan. 8. Press the ON/ZERO key to calibrate with the 200 gram mass. “C” will flash on the screen for a moment, then the screen will display “done” briefly before going into live measurement mode. 9. Remove the 200 gram mass. The 0 gram calibration step happens automatically and very quickly, so it may appear that the balance is skipping over it. 19 PART B: OPERATION 1. With the balance on, place a weigh boat, weigh paper, or small vessel (such as an Erlenmeyer flask) on the balance pan. 2. Press the ON/ZERO key to tare the weight of the weighing vessel. 3. Place the substance to be weighed into the weighing vessel and allow the value to stabilize on the display. The stability detector “*” will disappear when the reading is stable. 4. Record the mass of the substance, remove the weighing vessel and contents from the balance and turn off by pressing and holding the ON/ZERO key. 5. Check that the entire balance and the area around it are clean and dry. Reagents can be weighed directly into any vessel as long as it does not weigh more than 200 grams (the maximum capacity of this model). If weighing a chemical, use a clean, dry spatula to transfer it into the vessel, or if a free-flowing solid, gently sift it from the container. 20 SOP BSC-001 USE AND MAINTENANCE OF NU-AIRE BIOSAFETY CABINET Revision A, January 25, 2011 Title: Use and Maintenance of Nu-Aire NU-427 Biosafety Cabinet 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use of the Nu-Aire NU-427 Biosafety Cabinet. 2.0 SUMMARY OF METHOD MATERIALS 1. Nu-Aire NU-427 Biosafety Cabinet 2. 70% alcohol, paper towels PART A: OPERATION 1. This biosafety cabinet (“BSC”) is operated via a touch screen. To start up, firmly touch the screen in the top left corner. You will hear a beep and the BSC will start up after a few moments. 2. Touch the blower icon in the top left corner of the touch screen. The blower will come on and the icon will turn green. 3. Allow the cabinet to warm up for 15 minutes. 4. Touch the third icon from the left to turn on the fluorescent cabinet lights. 5. When the cabinet is ready to use, raise the window no higher than the maximum height (marked on the side of the cabinet) and clean the cabinet with alcohol. 6. Begin your work by wiping down your gloved hands and all materials to be placed in the hood, with 70% alcohol. 7. While working in the hood, avoid blocking the laminar flow by not placing any objects on top of the front grille. Keep a minimum of objects in the hood at all, to prevent blocking the COMMENTS 21 air flow. 8. As you work in the hood, keep sterile materials further back than nonsterile materials and avoid reaching over sterile containers, especially open containers of media. Refrain from excess talking and do not remove your hands from the cabinet until your work is finished. 9. When you are finished working in the hood, clean up materials (especially spilled nutrient medium that might grow contaminants) and wipe the work surface down with 70% alcohol. 10. Touch the blower icon to turn off the blower. Touch the UV light icon to turn on the UV light. This is on a timer so it will turn itself off after 15 minutes. The window must be completely closed for the light to turn on. PART B: MAINTENANCE 1. Every 12 months the hoods must be certified by a licensed technician. The technician will also replace HEPA filters and do any other preventive maintenance as needed. PART C: TROUBLESHOOTING If the blower icon is red or blue, there is a problem with the blower. An error message should appear on the screen. “Low inflow limit” means that there is a problem with the exhaust blower. Contact ACC Maintenance staff to check the blowers. 22 SOP CEN-001 USE OF EPPENDORF 5417R MICROCENTRIFUGE Revision A, April 22, 2011 Title: Use and Maintenance of Eppendorf 5417R Microcentrifuge 1.0 SCOPE AND APPLICATION: This SOP outlines the use and maintenance of the Eppendorf 5417R refrigerated microcentrifuge. 2.0 SUMMARY OF METHOD PROCEDURE COMMENTS 1. Turn centrifuge on with main power switch. 2. Press the LID button to open the centrifuge lid. 3. Open the rotor cover by rotating the center knob and pulling up on the cover. 4. Load tubes into centrifuge in a balanced configuration. This will reduce the risk that the centrifuge becomes unbalanced and vibrating. Additionally, samples may be thrown out if unbalanced, causing extreme damage. 5. Replace rotor cover. Press down on center knob and rotate to lock in place. 6. Close centrifuge lid and press down to lock in place. 7. Set temperature, time, and speed values to the appropriate settings using the cursor keys. To change speed units from “rpm” to “rcf”, press both cursor keys simultaneously. 8. Press the START key to begin the run. 9. If needed, press the STOP key to stop the run before the elapsed time. Allow the rotor to slowly decrease speed. 10. When the centrifuge can be safely opened, the OPEN key green LED will light up. 11. Press OPEN to open the lid and remove tubes. Do not open the lid until rotor stops. 23 SOP CEN-002 USE OF EPPENDORF 5804R CENTRIFUGE Revision A, May 31, 2010 Title: Use and Maintenance of Eppendorf 5804R Tabletop Centrifuge 1.0 SCOPE AND APPLICATION: This SOP outlines the use and maintenance of the Eppendorf 5804R refrigerated centrifuge. 2.0 SUMMARY OF METHOD PROCEDURE COMMENTS 1. Turn centrifuge on with main power switch (on right side of unit). 2. Press the power button (if off, it will be lit red; if on, it is lit green). 3. Press the LID button to open the centrifuge lid, if it is not already open. 4. Determine the correct rotor for your application. If it is installed, proceed to next step. If not installed, see Appendix for instructions on switching out the rotor. 5. Open the cover of the rotor by lifting off or rotating and lifting, depending on the design of the rotor. 6. Set temperature, time, and speed values to the appropriate settings using the cursor keys. To change speed units from “rpm” to “rcf”, press the speed button to change the symbol displayed. 7. You may need to wait for the centrifuge to cool to a chilled temperature before starting. If so, close the lid and then allow the centrifuge to cool down before loading. 8. Load tubes or plates into centrifuge rotor in a balanced configuration. 9. Replace rotor cover. 10. Close centrifuge lid and press down to lock in place; you will hear a click. Balance tubes and plates weighing an equal amount on opposite sides of the rotor. This will reduce the risk that the centrifuge becomes unbalanced and vibrating. Additionally, samples may be thrown out if unbalanced, possibly causing damage to the centrifuge and loss of samples. 24 11. Press the START key to begin the run. 12. If needed, press the STOP key to stop the run before the elapsed time. Allow the rotor to slowly decrease speed. 13. Press LID to open the lid and remove tubes or plates. 14. Replace the rotor cover and turn off centrifuge when finished. 15. It is best to leave the centrifuge lid open when not in use, to prevent microbial growth inside the chamber. APPENDIX: Changing rotors 1. Remove the lid of the rotor. 2. Insert rotor key (simply a hex/Allen wrench with handle) into the top of the rotor and rotate to unfasten. 3. Lift the rotor out of the centrifuge. The rotor may stick; wiggle the rotor while firmly lifting to unstuck. 4. Place the new rotor in the centrifuge, aligning the center with the post in the middle of the centrifuge. 5. Use the rotor key to fasten the rotor down. 6. Store the extra rotor safely, preferably inside a padded box. Do not open the lid until rotor stops. 25 SOP CEN-003 VWR CLINICAL 200 CENTRIFUGE Revision A, May 31, 2011 Title: Use and Maintenance of VWR Clinical 200 Centrifuge 1.0 SCOPE AND APPLICATION: This SOP outlines the use and maintenance of the VWR Clinical 200 centrifuge. 2.0 SUMMARY OF METHOD PROCEDURE COMMENTS 1. Turn centrifuge on with main power switch (on left underside of unit). 2. Press the OPEN button to open the centrifuge lid, if it is not already open. 3. Load 15-mL tubes into centrifuge rotor in a balanced configuration. 4. Close centrifuge lid and press down to lock in place; you will hear a click. 5. Set the speed by pressing the rpm/rcf key and using the up and down arrows to set the speed. To switch between the units of speed, press the rpm/rcf key twice. Balance tubes weighing an equal amount on opposite sides of the rotor. This will reduce the risk that the centrifuge becomes unbalanced and vibrating. Additionally, samples may be thrown out if unbalanced, possibly causing damage to the centrifuge and loss of samples. 6. Set the time by pressing the time key and using the up and down arrows. 7. Press the START key to begin the run. DO NOT OPEN THE LID UNTIL ROTOR STOPS. 8. If needed, press the STOP key to stop the run before the elapsed time. Allow the rotor to slowly decrease speed. 9. Press LID to open the lid and remove tubes. 10. Turn off centrifuge when finished. 11. It is best to leave the centrifuge lid open when not in use, to prevent microbial growth inside the chamber. 26 SOP CEN-004 USE OF VWR GALAXY 16D MICROCENTRIFUGE Title: USE AND MAINTENANCE OF VWR GALAXY 16D MICROCENTRIFUGE 1.0 SCOPE AND APPLICATION: This SOP outlines the use and maintenance of the VWR Galaxy 16D microcentrifuge. 2.0 SUMMARY OF METHOD PROCEDURE COMMENTS 1. Turn centrifuge on with main power switch (on back of unit). 2. Lift lid to open. 3. Load 1.5-2.0 mL tubes into centrifuge rotor in a balanced configuration. Replace rotor cover. 4. Close centrifuge lid. 5. Set the speed using the up and down arrows next to the speed display, up to 14,000 rpm. Toggle between rpm and g-force using the mode key. Balance tubes weighing an equal amount on opposite sides of the rotor. This will reduce the risk that the centrifuge becomes unbalanced and vibrating. Additionally, samples may be thrown out if unbalanced, possibly causing damage to the centrifuge and loss of samples. 6. Set the time using the up and down arrows next to the time display (in minutes). 7. Press the START key to begin the run. Lid will lock during the run and will not open until the rotor stops. 8. If needed, press the STOP key to stop the run before the elapsed time. Allow the rotor to slowly decrease speed. 9. The lid will unlock and the unit will beep when the run is finished. 10. Turn off centrifuge when finished. DO NOT OPEN THE LID UNTIL ROTOR STOPS. 27 SOP CUV-001 SELECTING, USING, AND CLEANING CUVETTES Revision A, March 31, 20 Title: Selecting, Using and Cleaning Cuvettes 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for selecting, using and cleaning cuvettes. 1.0 INTRODUCTION AND SELECTION OF CUVETTES Cuvettes are used as containers for spectroscopic and fluorometric readings and as such, must be optically clear and free from scratches and chemical contamination. Cuvettes can be made of a variety of materials, including glass, quartz, and plastic, and vary widely in quality, cost, and versatility. Materials The most expensive cuvettes are made of quartz and have very transparent optical qualities. These cuvettes are sometimes made in matched sets that can be used for a blank and a sample to minimize the error contributed by a difference in optical properties between cuvetttes. These are used for highly sensitive assays that are subject to error. Glass cuvettes are often used for visible range spectroscopy because high-quality glass is optically clear in the visible range. Plastic cuvettes are very inexpensive and are usually disposable. However, one must be very careful when selecting a plastic cuvette because many plastics are not transparent to UV light and also can autofluoresce, making them unsuitable for UV spectroscopy and fluorescence assays. Design For fluorescence, it is important that all four sides of the cuvette are optically clear because the excitation light will pass through one window, while the emission leaves at a 90º angle to it. This is unlike a spectrophotometry cuvette which needs to allow light to pass through in a straight line. Some spectrophotometry cuvettes are designed for very small samples, often as small as 50 uL. This allows the technician to load a small amount of a precious sample without having to dilute it to fill the cuvette. These are sometimes referred to as microcuvettes. Cuvettes designed like this are sometimes supplied sterile and nuclease-free so that the sample can be recovered without becoming contaminated. If the viewing window is isolated (i.e., the entire side of the cuvette is not transparent and there are some frosted areas), it is important that the light beam height is compatible with the viewing window height. In other words, the light beam must be able to pass through the viewing window. Sometimes the beam height of the instrument is higher or lower than the viewing window of the cuvette you have available. If the beam height is too low, nothing can be done and you will need to find another cuvette. However, if the beam height is too high, you may be able to insert a height adaptor into the sample compartment that brings the viewing window to the correct height. Note: Microplates used for optical experiments in a microplate reader can be selected using much of the same criteria as above. Microplates are all plastic and so the optical clarity and fluorescence properties must be considered when selecting a plate. Fluorescence assays are often done using opaque white or black plates, which reduces the transfer of signal between wells. 28 2.0 SUMMARY OF METHOD PROCEDURE 2.1 USING CUVETTES 1. Select an appropriate type of cuvette for the optical method and instrument you need to use. Consider the material and design of the cuvette when making your selection (see introduction). 2. If using previously used cuvettes, make sure that they are clean inside and free from water spots or scratches, especially where the light beam will pass through. 3. Fill cuvettes with adequate liquid so that the light beam will pass through the liquid. This can vary widely depending on the cuvette design. Usually the package will define the minimum sample volume. 4. Wipe the viewing sides of the cuvette with a lint-free wipe before loading into the sample compartment to remove fingerprints or dust. Always handle cuvettes with gloved hands to minimize fingerprints and hold cuvettes away from the viewing windows. 2.2 CLEANING CUVETTES 1. Cuvettes to be reused should never be allowed to dry out because this leaves a residue that is very hard to clean off. Sink used cuvettes in a container of deionized water immediately after using. Leave to soak for no more than 24 hours before draining out the water. 2. Rinse the inside of each cuvette with 70%. Use a squirt bottle. 3. Rinse each cuvette thoroughly with deionized water. 4. Place cuvettes upside down on a test tube drying rack or on top of an absorbent towel. Do not leave the COMMENTS 29 cuvettes to dry inside a beaker or other deep container, since they will take too long to dry and will also retain dried residue from the water. 5. Reuse disposable glass and plastic cuvettes no more than three times before discarding. Quartz cuvettes can be reused again and again until they break or are badly scratched. 30 SOP DTR-001 DIGITAL TEMPERATURE RECORDER Revision A, June 8, 2011 Title: Use and Maintenance of Digital Temperature Recorder 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use of a digital temperature recorder. 2.0 SUMMARY OF METHOD MATERIALS 1. Digital temperature recorder 2. Probe PART A: OPERATION 1. Insert the temperature probe into the recorder. 2. Press the round yellow power button on the recorder to operate. Timer will start when unit is turned on. 3. Situate probe in the area in which you want to measure temperature. Close the door to freezers, incubators, etc, on the cord gently so you do not damage the cord. 4. Allow the probe to equilibrate to the temperature. This may take a few minutes. 5. Record temperature for the desired time period. 6. Remove probe and turn off recorder. 31 SOP ELC-002 MINI-PROTEAN CELL Revision A, February 22, 2011 Title: Operation of Bio-Rad Mini-Protean Vertical Electrophoresis System 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the Bio-Rad Mini-Protean vertical electrophoresis system (“cell”). 2.0 INTRODUCTION The Mini-Protean cell is designed for fast and simple vertical electrophoresis using polyacrylamide gels. Precast Bio-Rad Ready Gels are recommended; handcast gels require a casting apparatus that we do not have in our lab. One or two gels can be run at a time. 3.0 SAFETY Do not operate the Mini-Protean Cell at a voltage higher than 600 volts. Before use, inspect the tank for cracks or chips, which may allow the buffer to leak from the tank and cause a potential electrical hazard. Additionally, inspect all electrical cables, banana jacks, and plugs for loose connections, cracks, breaks, or corrosion. Do not use any part that is cracked, charred, or corroded. These parts may also cause a potential electrical hazard. Contact your local Bio-Rad representative before using a part that may be considered hazardous. During electrophoresis, inspect the base and workbench for any signs of buffer leakage. If leaking buffer is detected, disconnect the power to the cell immediately and contact your local Bio-Rad representative. 4.0 SUMMARY OF METHOD MATERIALS 1. Mini-Protean Cell (mini tank, assembly, and lid) 2. Precast polyacrylamide gel 3. 150V power supply 4. 1X gel running buffer 5. Sample loading buffer 6. Samples to be loaded 7. Micropipettor and gel loading tips PART A: GEL PREPARATION AND ASSEMBLY 1. Remove the Ready Gel from the storage pouch. 2. Gently remove the comb. Pull off the tape at the bottom of the Ready Gel cassette to expose the bottom edge of the gel. 3. Set the clamping frame to the open 32 position on a clean flat surface. 4. Place the first gel sandwich or gel cassette (with the short plate facing inward) onto the gel supports; gel supports are molded into the bottom of the clamping frame assembly; there are two supports in each side of the assembly. 5. Now, place the second gel on the other side of the clamping frame, again by resting the gel onto the supports. 6. Using one hand, gently pull both gels towards each other, making sure that they rest firmly and squarely against the green gaskets that are built into the clamping frame; make certain that the short plates sit just below the notch at the top of the green gasket. It is critical that gel cassettes are placed into the clamping frame with the short plate facing inward. Also, the clamping frame requires 2 gels to create a functioning assembly. If an odd number of gels (1 or 3) is being run, you must use the buffer dam. 7. While gently squeezing the gel sandwiches or cassettes against the green gaskets with one hand (keeping constant pressure and both gels firmly held in place), slide the green arms of the clamping frame over the gels, locking them into place. 8. The arms of the clamping frame push the short plates of each gel cassette up against the notch in the green gasket, creating a leak-proof seal (check again to make certain that the short plates sit just below the notch at the top of the green gasket). At this point, the sample wells can be washed out with running buffer, and sample can be loaded. PART B: RUNNING THE GEL 1. Place the assembled gel(s) into the tank. The assembly should be inserted into the tank on the side away from you, with the black electrode on the left. 2. Pour 1X running buffer into the The lid only goes on one way, so it is important to insert the assembly correctly. You do not want to have to move it after the gels are loaded with samples. 33 assembly (upper chamber). Before filling the rest of the tank, make sure the assembly does not leak. If it does, you must remove the assembly and assemble it correctly. 3. Fill the upper chamber with buffer until the level is just below the outer plate(s) of the gel(s). Fill the tank up to the 2-gel level marker (requires about 700 mL). 4. Make sure the wells are all filled with buffer. Use a pipet to blast out any stubborn air bubbles. This step also removes unpolymerized acrylamide from the wells, which can interfere with protein migration. 5. Load samples and standards into the wells using a gel loading pipet tip or syringe. Be careful not to poke a hole in the gel or blast samples out when loading (stop just short of the second stop). 6. Place the lid on the base, matching the electrode colors. 7. Connect the electrical leads to a power supply. Set to 200 volts and press start. 8. Check the area around the electrodes to make sure tiny bubbles are forming. This is evidence of an electrical current in the buffer. 9. Periodically check the system to make sure the tracking dye is migrating in the gel. Do not allow the tracking dye to migrate off your gel. Do not allow the buffer to heat above 40ºC or your gel could melt. 10. When you are finished running the gel, stop the power supply and disconnect the leads. 11. Remove the lid from the base and take out the assembly. Pour the buffer out before disassembling. Be careful not to jostle or move the base after loading wells because you will lose your sample(s). 34 12. Remove the gel from the assembly. 13. Remove the gel from its cassette by separating the two plates of the cassette. This can be done easily by inserting a spatula between the plates and twisting. 14. Remove the top plate and invert the other plate in a container of water, buffer, or staining solution. Agitate gently until the gel falls off the plate. 15. Fix and/or stain the gels as desired. 16. Visualize the gel using the appropriate light source (white light for Coomassie blue stain). 17. Discard the electrophoresis buffer and gel. 18. Rinse the lid, tank, and assembly with tap water and dry completely before storing. Be extremely careful with polyacrylamide gels, which are very fragile and rip easily. 35 SOP ELC-003 MINI TRANS-BLOT TRANSFER CELL Revision A, June 8, 2011 Title: Operation of Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell. 2.0 INTRODUCTION The Mini Trans-blot cell is part of Bio-Rad’s modular Mini-Protean Tetra system for electrophoresis and blotting. The cell fits into the Mini-Protean tank and can be used for electrophoretic transfer of species from a gel onto a membrane. 3.0 SAFETY Before use, inspect the tank for cracks or chips, which may allow the buffer to leak from the tank and cause a potential electrical hazard. Additionally, inspect all electrical cables, banana jacks, and plugs for loose connections, cracks, breaks, or corrosion. Do not use any part that is cracked, charred, or corroded. These parts may also cause a potential electrical hazard. Contact your local Bio-Rad representative before using a part that may be considered hazardous. During operation, inspect the base and workbench for any signs of buffer leakage. If leaking buffer is detected, disconnect the power to the cell immediately and contact your local Bio-Rad representative. 4.0 SUMMARY OF METHOD MATERIALS 1. Mini-Protean tank and lid 2. Mini Trans-blot cell 1. Electrode module 2. Gel holder cassette 3. Fiber pads (2) 4. Cooling unit 3. Filter papers (2) 4. Membrane 5. Gel containing separated samples 6. Roller or glass test tube 7. 200V power supply 8. 1X transfer buffer (refrigerated) PART A: PREPARATION FOR BLOTTING 1. The cooling unit should be filled with water and frozen before use. Chilling the transfer buffer before using will also help maintain a cool temperature during the transfer. 36 2. Cut the membrane and filter paper to the dimensions of the gel, if necessary. 3. Equilibrate the gel and soak the membrane, filter paper, and fiber pads in transfer buffer (15-20 minutes). Always wear gloves while handling membranes. Be extremely careful with polyacrylamide gels, which are very fragile and rip easily. 4. Prepare the gel sandwich: a. Place the cassette, with gray side down, on a clean surface. b. Place one pre-wetted fiber pad on the gray side of the cassette. c. Place a sheet of filter paper on the fiber pad. d. Place the equilibrated gel on the filter paper. e. Place the pre-wetted membrane on the gel. f. Place a piece of filter paper on the membrane. g. Place a fiber pad on the filter paper. Remove any air bubbles under the gel or membrane by rolling them out. Use a specialized roller or simply a glass test tube. 5. Close the cassette firmly, being careful not to move the gel and filter paper sandwich. Lock the cassette closed with the latch. 6. Place the assembled cassette into the module. 7. Place the module into the tank with the black electrode to the top and left. Add a cooling unit to the tank. Fill the tank with buffer to the “blotting” mark on the tank. 8. Add a small stir bar to the tank and place the tank on top of a stir plate. Set the stirring speed as fast as possible without splashing. Stirring the buffer will help maintain a cool and even buffer temperature and homogeneous ion concentration. 9. Place the lid on the tank, matching the electrode colors. The lid only fits on one way, so make sure the module is oriented correctly. 37 PART B: RUNNING THE GEL 1. Connect the electrical leads to a power supply. Set to an appropriate voltage and current and run the transfer. 2. After starting, check the area around the electrodes to make sure tiny bubbles are forming. This is evidence of an electrical current in the buffer. 3. At the end of the run, turn off the power supply and disconnect the leads. 4. Remove the lid from the tank. Remove the module from the tank and the cassette(s) from the module. 5. Disassemble the cassette(s) and set aside the membrane. Discard the transfer buffer, filter papers, and gel. 6. Detect species on the membrane using the appropriate detection method. 7. Rinse the lid, tank, fiber pads, cassettes, and module with tap water and dry completely before storing. The voltage, current, and time should be selected based on the type of buffer, gels, and analyte(s). One option for SDS-PAGE gels containing proteins in TG buffer with alcohol would be a one-hour transfer at 100 V and 250 mA. 38 SOP ELC-004 EDVOTEK ELECTROPHORESIS CHAMBERS (M12 AND HEXAGEL) Revision A, June 25, 2012 Title: Operation of Edvotek Horizontal Electrophoresis Chambers 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the Edvotek M12 and Hexagel horizontal electrophoresis chambers. 2.0 INTRODUCTION The Edvotek system is designed for fast and simple horizontal electrophoresis using agarose gels. The gel is cast outside the base chamber using the included tray, combs, and bumpers for casting. 3.0 SAFETY Do not operate the apparatus at a voltage higher than 300 volts. Before use, inspect the box base for cracks or chips, which may allow the buffer to leak from the base and cause a potential electrical hazard. Additionally, inspect all electrical cables, and plugs for loose connections, breaks, or corrosion. Do not use any part that is cracked, charred, or corroded. These parts may also cause a potential electrical hazard. Contact your laboratory technician. During electrophoresis, inspect the base and workbench for any signs of buffer leakage. If leaking buffer is detected, disconnect the power to the cell immediately and contact your laboratory technician. 4.0 SUMMARY OF METHOD MATERIALS 1. Edvotek electrophoresis chamber (M12 or Hexagel) 2. Gel casting equipment 1. Bumpers (2 per tray) 2. Casting tray (7x7 cm or 7x14cm) 3. Comb (6, 8 or 10 well) 3. Electrophoresis power supply 4. Agarose powder 5. 1X Running buffer such as TAE or TBE 6. Sample buffer/loading dye 7. Samples to be loaded 8. Micropipettor and tips 39 PART A: GEL CASTING 1. Prepare 1X buffer and molten agarose. The 7x7cm casting trays hold approximately 30mL of molten agarose, and the 7x14cm hold about 60mL. 2. Cool the molten agarose to 50-60ºC. 3. Press the black bumpers onto either side of the casting tray to form a tight seal. This is easiest if you place the bumper slit up on the bench and press the tray down onto it. Warning: Hot agarose (>60°C) may cause the tray to warp or craze and will decrease the lifetime of the tray. Warping may also result in sample wells of uneven depth. 4. Select a comb (blue or white) with the appropriate number of wells, and insert it into the tray. 5. Pour about 30mL of agarose into the 7x7 tray (60mL into the 7x14.) Check for bubbles or debris and remove these with a transfer pipet before the agarose cools. 6. Allow 20-40 minutes for the agarose to cool and solidify. 7. Carefully remove the comb and bumpers from the gel. To avoid ripping the gel, it can be helpful to separate the gel from the bumpers and tray using a slim spatula before attempting to remove. PART B: RUNNING THE GEL 1. Place the casting tray with solidified gel into the electrophoresis chamber, orienting the wells toward the black electrode. The tab on the side of the tray will fit into a cut out on the side of the chamber. 2. Pour 1X electrophoresis running buffer over the gel until it is covered by 2-6 mm. 3. Make sure the wells are all filled with buffer. Use a pipet to blast out any stubborn air bubbles. 4. Load samples and standards (premixed with sample buffer/loading dye to a final concentration of 1X) into the wells using a regular pipet tip. Be careful not to poke a hole in the gel or blast samples out when loading (stop just short of the second stop). The M12 chamber requires about 400 mL buffer, while the Hexagel needs 2-3 times that amount. 40 5. Place the lid on the base, matching the electrode colors (black to black and red to red.) 6. Connect the electrical leads to a power supply and choose an appropriate voltage, usually between 75 and 150 volts. Press start. Be careful not to jostle or move the base after loading wells because the sample(s) can float out. Edvotek DuoSource power supplies can be set to either 75 or 150 volts. Other power supplies can be adjusted to more specific voltages. 7. Check the area of buffer around the electrodes to make sure tiny bubbles are forming. This is evidence of electrical current passing through the buffer. 8. Periodically check the system to make sure the tracking dye (in the sample buffer) is migrating down the gel. Do not allow the tracking dye to migrate off your gel. Do not allow the buffer to heat above 40ºC or your gel could melt. If the chamber is very warm to the touch or excessively steamy, you may be overheating the system. Voltage is directly related to heat. If your gel is becoming hot, reduce the Voltage so as not to melt the gel. 9. When you are finished running the gel, stop the power supply and disconnect the leads. 10. Remove the lid from the base. Carefully take out the gel in the tray and place it in a container for transport to the gel documentation system. 11. Discard the electrophoresis buffer and gel, treating as hazardous waste if necessary. 12. Rinse the lid, base, tray, comb, and bumpers with tap water and dry completely before storing. If agarose residue remains on any of the casting parts, wash gently with soapy water and a brush, rinse thoroughly, and dry. Avoid scrubbing or aggressively drying the inside of the chamber because the platinum electrodes are delicate and can be ripped out easily. A gentle tap water rinse and blot-dry with a paper towel is sufficient and will not damage the electrodes. 41 SOP ERP-001 USE OF EPPENDORF REPEATER PLUS PIPETTOR Title: Operation and maintenance of Eppendorf Repeater Plus Pipettor 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for operation and maintenance of the Eppendorf Repeater Plus pipettors. 2.0 INTRODUCTION The Repeater Plus forms a dispensing system for repetitive pipetting when used in conjunction with Eppendorf Combitip Plus Tips. A supply of liquid is aspirated into the Combitip Plus and then dispensed step by step. The built-in electronics of the device automatically recognize the Combitip Plus inserted and the position of the volume selection dial, and the current pipetting volume appears in the display. Using the volume selection dial, 20 different volumes can be selected for each Combitip Plus. The smallest pipetting volume can be dispensed a maximum of 100 times. The largest pipetting volume can be dispensed a maximum of 5 times. 3.0 SUMMARY OF METHOD PART A: OPERATION COMMENTS 1. Determine the volume you would like to dispense, and choose a Combitip. 2. Insert the Combitip into the pipettor from the bottom; it will click into place. 25- and 50-mL tips require an adaptor. 3. The selected volume will appear on the display. To change the selected volume, rotate the dial left or right. 4. Push the filling lever all the way down. 5. Insert the tip into the liquid to be dispensed. Pull the filling lever up all the way while keeping the tip submerged. 6. Press the pipetting lever once to discard the first measurement (not accurate). 7. Press the pipetting lever and the correct volume will be dispensed. 8. Refill tip if necessary by repeating Step 5. 9. When you are finished dispensing, discard any leftover fluid by pressing Always keep the micropipettor in vertical position to ensure that no liquids enter the micropipetter or drip from the disposable tip. 42 filling lever all the way down. 10. Eject tip by pressing on the ejection keys at the sides of the pipette. 43 SOP FLU-002 VARIAN CARY ECLIPSE FLUOROMETER Revision A, June 8, 2011 Title: Operation of the Varian Cary Eclipse Fluorometer 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for operation of the Varian Cary Eclipse fluorometer. 2.0 INTRODUCTION The Cary Eclipse fluorescence spectrophotometer can read fluorescence intensity at defined wavelengths, scan a sample for emission and excitation maxima, perform kinetics assays, and can also be used to read phosphorescence and chemiluminescence. The xenon flash lamp is long-lasting and never needs replacing; excitation and emission wavelengths are tunable to 1 nm. The instrument is operated through an attached computer with Eclipse software. 3.0 SUMMARY OF METHOD COMMENTS PART A: MATERIALS 1. Varian Cary fluorometer 2. Laptop computer with Windows XP OS and Eclipse software installed 3. Fluorometry cuvettes (optically clear on all four sides) The Eclipse software has not been tested with Windows 7, so be sure your attached computer has Windows XP. PART B: SCANNING For determining emission and excitation maxima for an uncharacterized sample, you will want to use the Scan module. 1. Turn on the fluorometer using the on/off switch on the bottom front of the unit. A yellow LED will come on while the instrument calibrates; wait about 2 minutes until calibration is over and the light will turn green. 2. Connect the flurometer to a laptop via USB cable and turn on the computer. Click on the Cary Eclipse folder on the desktop and then select the Scan icon. 3. Insert sample. Open the sample compartment by sliding the lid back. Place a fluorometry cuvette containing your sample in the compartment and close the lid. A cuvette with frosted sides will not work because the light paths in and out of the cuvette are at a right angle. All four sides must be optically clear and made of lowfluorescence plastic. 44 4. Select Open Method from the File menu to load a previously defined method. If you don’t have a method, click the Setup button. Select the Excitation or Emission button, and enter wavelengths for excitation and emission and the scan speed. Select the Options tab, and select PMT voltage. Start with Medium. Click OK. 5. When you have loaded a previous or new method, you will see the main screen. Click on the traffic light icon (“Start”) to begin reading your sample using the selected method. You will be prompted to name your sample; do so and click OK. The scan will begin and you will see the wavelength changing in the upper right corner. 6. When the scan is complete you will see a fluorescence spectrum. 7. If spectrum intensity is weak or clipped, go back to Setup and adjust PMT voltage and any other parameters desired. Higher PMT voltage and larger slits give higher sensitivity. 8. Remove the sample from the compartment. Load another sample and repeat the process. 9. When finished, remove the last sample from the compartment and close the lid. Turn off the fluorometer and the computer. Rinse your cuvettes with deionized water and/or alcohol and place upside down to dry. 45 SOP FPLC-001 BIO-RAD DUOFLOW FPLC SYSTEM Revision B, April 25, 2011 Title: Use and Maintenance of the Bio-Rad DuoFlow Fast Liquid Chromatography System 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Bio-Rad DuoFlow Forward Phase Liquid Chromatography (FPLC) System. 2.0 INTRODUCTION The Bio-Rad DuoFlow FPLC system offers a simple yet versatile way to do liquid chromatography. This system is equipped to perform forward phase liquid chromatography at medium pressure. The system consists of several components: Workstation with dual-piston F10 pumps Maximizer mixer AVR7-3 automated injection valve Dual wavelength UV detector Conductivity and pH monitors BioFrac fraction collector These components can be operated manually or controlled using the BioLogic software on an attached computer. This SOP will describe how to operate using BioLogic. The DuoFlow F10 pumphead operates at up to 20 mL/min and 3500 psi when used with the Maximizer. 3.0 SUMMARY OF METHOD MATERIALS 1. FPLC system (list of components in Introduction) 2. Filtered and degassed buffer and salt solutions and water 3. Injection syringe and flat tipped needle (both Luer-lok) 4. Chromatography column 5. Sample 6. Waste container (large beaker, flask, or bottle) PART A: STARTUP 1. Select the appropriate column, buffers, and salt solution for your analysis. Filter (to at 0.2 µm) and degas buffers and deionized water. 2. Turn on the Maximizer, workstation pumps, and fraction collector using the power toggle switches on the front or back of each component. 46 3. Open the BioLogic software on the attached computer. 4. When the system is first started up for the day or after a long storage, you must prime the pumps in order to remove any air. 5. Make sure that the column is not in-line before proceeding! The column is installed after the sample inject valve and before the UV flow cell. 6. Submerge all four inlet tubing lines in pure water. Secure all three waste lines (narrow, translucent white tubing) in a large waste container. Check the manual screen for the following system components: Maximizer and gradient pump, fraction collector, UV detector, and workstation valve AVR7-3. You may need to click the arrow at the top right of the valve window to view the AVR7-3 valve. The column may become damaged from high pressure during priming or purging, so it should be removed from the line before these steps. 7. In the software, place the Maximizer in Local mode (so you can control it manually). 8. Attach a large Luer-lok syringe (20mL or larger) to the port on pump A. Twist left and draw out the syringe. The first pull may just be air. Twist the port closed (with syringe attached!) and then remove the syringe and expel air and liquid. Repeat once or twice more until the syringe contains mostly liquid. 9. Repeat step 5, but this time, press the port select button below to select A2. 10. Repeat steps 5 and 6 on pump B and make sure to prime both B1 and B2 using the port select button. 11. The next setup step is to purge the system. In this step, you flush the entire system with clean eluent. This is especially important if you had the system stored in ethanol or sodium azide. In this case, you should flush with water only to avoid salt precipitation. 12. In the software, place the Maximizer in System mode so you can control using BioLogic. Locate the AVR7-3 valve and click “P” to place the valve in the Purge position (you will hear the valve If you remove the syringe before closing the port, air will enter the pump. 47 move). 13. Place the Maximizer in Local mode again. Press the Purge buttons A and B on the front of the pump (lights will flash green). Wait two minutes, then press the valve port select buttons on the front of the Maximizer to select the other two ports. Purge those for two minutes more. Press the purge buttons to stop the pump. 14. Place the Maximizer in System mode and the AVR7-3 valve in the Load position by clicking “L”. Set the pump flow rate at 1.0 mL/minute and start the pump. Water will flow through the entire system and out of the fraction collector. 15. Before the first run of the day, calibrate the pH electrode. Unscrew the pH monitor to expose the electrode and place it in pH 7 standard buffer. 16. From the Utilities menu, select pH probe calibration. Press Set. When the reading has stabilized, click OK. 17. Rinse the electrode with deionized water. 18. Repeat with pH 10 standard buffer. 19. Reassemble the pH monitor. 20. Before the first run can be initiated, the system and column should be equilibrated. 21. Submerge the inlet tubing lines in the appropriate buffer(s), salt, and water. a. A1 (red): acid (such as Tris-HCl) b. A2 (blue): base (such as Tris base) c. B1 (yellow): water d. B2 (green): salt 22. Prime and purge the pumps as before. 23. Place the column in line. First remove 48 the union that connects the line, then remove the end caps from the stored column and install where the union was, making sure you orient it so the flow will pass through in the direction of the arrow on the column. 24. In the Manual screen of the software, set the pressure limits to the recommended values for the column. 25. Set the inject valve position to Load (L). Set the flow rate to 2.0 mL/minute. Set the buffer recipe by pressing the Setup button on the toolbar. Select the appropriate buffer pH and salt concentration for your protocol. 26. Wash the column with equilibration buffer conditions and 100% B (highest possible salt concentration) for about 3 minutes. 27. Equilibrate the column with the same buffer but change to 0% B for about 7 minutes. 28. The system is now ready to run a separation. PART B: OPERATION 1. Open the Browser tab to locate a saved method, filed under different Users. Double-click the method and the protocol will open. 2. Alternatively, create a new method. Click New Method on the toolbar and you will be prompted to pick a user and name the method. 3. A method editor will open. On the left side of the screen you will be able to add instrument components to the method. These should be added: Workstation Mixer AVR7-3 valve UV detector Conductivity Setting the upper pressure limit will protect the column from excessive pressure that could damage it. 49 pH monitors Fraction collector 4. You also need to design a protocol for the method. Click the protocol icon on the toolbar and an editor will open. Add steps such as turning on the UV lamp, zero baseline, isocratic flow, inject sample, and linear gradient to create your protocol. Make sure to equilibrate before the injection and after the run is over. 5. When you have created and/or opened the method you need, you may begin. Click Run on the toolbar and the Run screen will appear. 6. Load sufficient 12 mm tubes into the fraction collector. 7. Place the inject valve in the Load (L) position. Draw up a volume at least 10% more than the sample loop installed into a Luer-lok syringe with a flat-tipped needle. 8. Place the needle in the inject port and fill the loop, injecting more than the loop volume to rinse it out. Do not remove the needle. 9. Click the green Start button on the toolbar to launch the run. The sample will be injected automatically at the correct step in the protocol. 10. As the run progresses, each step in the protocol will acquire a checkmark as it is completed. The Run screen displays three traces: a. UV absorbance—shows species as they leave the column b. Conductivity—tracks the progress of a salt gradient c. pH—tracks the progress of a pH gradient 11. When the run is finished, the pumps will automatically stop and a Run Finished message appears at the bottom right 50 of the status bar. PART C: SHUTDOWN 1. After the last run of the day, make sure that no sample is left in the injector or column. Each method should include a step that washes sample off the column so it is clean for the next run, as well as a final equilibration step. If this is done with the last run of the day, there is no need to further wash the system. High salt buffer should not be left in the system overnight. 2. Wash behind each pumphead with about 15 mL of water. 3. If the instrument is to be stored longer than one week without using, you must flush all buffers out of the system and replace with an antimicrobial solution. 4. First, remove the column and replace with a union. Inject the appropriate antimicrobial solution through the column using a Luer-Lok syringe and cap both ends (see column manufacturer instructions). 5. Place the inlet tubing into filtered, degassed deionized water. Pump water through the system at 2 mL/minute for about 5 minutes. 6. Wash behind each pumphead with about 15 mL of water. 7. Transfer the inlet tubing into filtered 20% ethanol or 0.05% sodium azide and pump through the system at 2 mL/minute for about 5 minutes. 8. When the system is full of antimicrobial solution, remove all eluent bottles and turn off each component. PART D: TROUBLESHOOTING If you see a sawtooth pattern in the pump pressure trace, you most likely have air bubbles in the system. This is due to the 51 compressability of air compared to water. Purge your pumps and start over. 52 SOP GDS-003 CHEMIDOC GEL DOCUMENTATION SYSTEM WITH QUANTITY ONE Revision A, June 8, 2011 Title: Use of ChemiDoc Imaging System with Quantity One Software for Gel Documentation 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for using the Bio-Rad ChemiDoc imaging system with Quantity One software for gel documentation. 2.0 SUMMARY OF METHOD PART A: UV IMAGING OF ETBR- OR SYBR SAFE-STAINED GELS 1. Turn on the system using the on/off switches on the A/C power supply and on the back of the unit. To consult the instruction manual, doubleclick on the Quantity One Instruction Manual icon on the desktop. 2. Open the software by double-clicking on the Quantity One icon on the desktop. 3. In the file menu, click on ChemiDoc XRS. A new window will open. Under Select Application, make sure that UV Transillumination is listed as the application. If it says something else, such as “White Transillumination,” click Select and choose UV Transillumination. 4. Position the gel(s) on top of the UV light box and close the door and/or drawer of the unit. Press the UV Trans button on the front of the unit. 5. Click on Live View and an image of the gel will appear. Change the iris, zoom, and focus settings until a satisfactory image is obtained. Click on Freeze. This will activate the Acquire Image options. 6. Click on Manual Expose. Wait a moment for the image to appear in a separate window. If the image is too light or dark, or if there is saturation in the image (shown in red), change the exposure time setting and expose again. Reposition the gel in the drawer if necessary. The UV light will automatically switch off, and you will need to press the UV Trans button when you close the drawer. If you make an exposure, the UV light will 53 7. If the image obtained is satisfactory, you may save and print. To print, click the print icon on the toolbar while the image is in the active window. To save, click the “X” in the top right corner of the image window, then choose Save. shut off. If you decide to make another exposure, you will have to turn the UV light back on by pressing the UV Trans button again. You must choose Mitsubishi P93D printer to print to the thermal printer. 8. When you are finished imaging, remove the gel from the drawer and wipe the surface clean with a paper towel. Turn the ChemiDoc off at both power switches. PART A: VISIBLE LIGHT IMAGING OF GELS 1. Turn on the system using the on/off switches on the A/C power supply and on the back of the unit. Note: visible light imaging with the ChemiDoc is currently done using a white light converter screen on top of the UV light box. 2. Open the software by double-clicking on the icon on the desktop. 3. In the file menu, click on ChemiDoc XRS. A new window will open. Under Select Application, make sure that UV Transillumination is listed as the application. If it says something else, such as “White Transillumination,” click Select and choose UV Transillumination. 4. Place the white light converter screen on top of the UV transilluminator inside the cabinet. 5. Position the gel(s) on top of the converter screen and close the door and/or drawer of the unit. Press the Trans UV button on the front of the unit. 6. Click on Live View and an image of the gel will appear. Change the iris, zoom, and focus settings until a satisfactory image is obtained. Click on Freeze. This will activate the Acquire Image options. 7. Click on Manual Expose. Wait a moment for the image to appear in a separate window. If the image is too light or dark, or if there is saturation in the image (shown in red), change the Reposition the gel in the drawer if necessary. The UV light will automatically switch off, and you will need to press the UV Trans button when you close the drawer. 54 exposure time setting and expose again. 8. If the image obtained is satisfactory, you may save and print. To print, click the print icon on the toolbar while the image is in the active window. To save, click the “X” in the top right corner of the image window, then choose Save. 9. When you are finished imaging, remove the gel from the drawer and wipe the surface clean with a paper towel. Turn the unit off at both power switches. If you make an exposure, the UV light will shut off. If you decide to make another exposure, you will have to turn the UV light back on by pressing the UV Trans button again. You must choose Mitsubishi P93D printer to print to the thermal printer. 55 SOP GDS-004 CHEMIDOC IMAGER WITH IMAGE LAB SOFTWARE Revision B, July 12, 2012 Title: Use of Bio-Rad ChemiDoc Imager Using Image Lab Software 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use of the Bio-Rad ChemiDoc Imager with Image Lab software for gel documentation. 2.0 SUMMARY OF METHOD MATERIALS 1. ChemiDoc Imager 2. Attached computer with Image Lab software 3. Gels to be imaged PART A: OPERATION 1. Turn on the cabinet (switch is on the back left of the cabinet) and the camera power supply (black box). 2. Double-click the Image Lab icon on the desktop to open the software. 3. You may use an existing protocol by choosing Open under the File menu. Proceed to step 9. 4. To create a new protocol, click New Protocol on the menu bar. Under Gel Imaging, select the application you need (nucleic acid or protein gel) and select the stain you are using. 5. You may also select a gel type from a drop-down menu; this can be helpful if you are using the ChemiDoc to analyze your gel results. You may also enter the image area manually. 6. You can select optimization for either faint or intense bands, or you can manually select the exposure time. 7. You may also select the color you want the image displayed in, which should be the same as your stain. Please note, Important: the Mitsubishi P93D thermal printer will only work on a computer using Windows XP. Windows 7 will not allow the printer driver to be installed. Image Lab works fine with Windows 7, but images will not print. 56 the printer will only print in black and white. 8. If you would like to include an image analysis and/or a report in your protocol, check the boxes next to these and select parameters for Lane and Band Detection, Molecular Weight Determination, and the type of output for a report. These steps are optional. 9. When you are ready to run the protocol, click the yellow Position Gel button. Open the drawer on the cabinet and place your gel on either the UV light box or on top of the white conversion screen (if you are using white light illumination). For either UV or white light (with the conversion screen), you must have the filter in position 1. 10. Position the gel in the center of the box and close the drawer. You may zoom in or out by moving the camera meter below the image. When the image appears how you want it to, click the green Run Protocol button. This will initiate image collection. 11. After the exposure your image will appear in a new window. Go to File > Save to save the image. If you need to open the file in another program such as Microsoft Paint, you will need to export the file as a JPEG. Do this by going to File > Export > Displayed Image and save the image as a JPEG. 12. To print, go to File > Print > and click Print. Make sure the Mitsubishi P93D is selected as the printer. This is a thermal printer that uses glossy paper and prints small images. When the image has printed, pull against the cutting edge to detach your photo. 13. When you are finished with the ChemiDoc, wipe buffer and gel residue off the light box with a paper towel. Turn off the camera and the cabinet. Another option is checking or unchecking the box next to “Highlight Saturated Pixels.” When checked, the display will show red highlights over pixels that are saturated (when the camera was at its max light recording ability). These saturated pixels indicate a high level of fluorescence or intense color in that region of the gel. Unchecking the box will hide the highlights. 57 PART B: TROUBLESHOOTING GUIDE 1. 1. If the ChemiDoc instrument is not detected by the software, make sure that BOTH the cabinet and the camera power supply are turned on. Lights should be visible on the front of the cabinet if it is on. 2. 2. If you see a bright circle on the image, check to make sure the flat field lens is not in position 2 of the filter wheel. This lens is white on top and pink on the bottom and is placed in position 2 during a calibration step. It should be removed after calibration, but if it was mistakenly left on, it can cause a circular reflection on gel images. 3. 3. If the printed images are an odd size or shape, make sure the print size is 1280x1280. Go to File > Print > Page Setup and choose 1280x1280 as the page size. 58 SOP GWC-001 CLEANING GLASSWARE Revision B, July 12, 2012 Title: Cleaning Glassware 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for cleaning used glassware and plasticware. 2.0 SUMMARY OF METHOD PROCEDURE COMMENTS 1. Remove any tape or labels from the glassware and remove any pen marks with acetone or ethanol. Use of this SOP indicates that the user has disposed properly of any hazardous materials contained in the dirty glassware. 2. Fill a wash tub with warm tap water and a few mL of Micro-90 detergent. Use a flat razor scraper to remove tape and labels, if necessary. 3. Soak dirty glassware for a few minutes before scrubbing thoroughly with a brush. 4. Rinse thoroughly with tap water three times. 5. Rinse thoroughly with deionized water three times. 6. To dry, hang glassware on pegboard if possible, or place upside down over paper towels or Labmat. If a stubborn chemical residue persists, soak the glassware in tap water and use a brush or a spatula to remove the residue (only if nonhazardous). Glassware with persistent residue of a hazardous chemical should be marked as hazardous waste (be sure to name the chemical). 59 SOP HPLC-001 Waters Breeze HPLC System Revision A, April 21, 2011 Title: Use and Maintenance of Waters Breeze HPLC System 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Waters Breeze HPLC System. 2.0 SUMMARY OF METHOD MATERIALS Waters Breeze HPLC System PART A: OPERATION 1. Turn on the HPLC pump, column heater, absorbance detector, and degasser. Wait for them to initialize and run self-diagnostic tests. 2. Turn on the computer attached to the HPLC. 3. Open the Breeze software and allow it to run through its system startup routine. 4. Minimize the Help window, if necessary. The sample queue workspace should be completely visible. 5. Click View Method on the command bar. Click on the open icon to open a method, or click the new icon to create a new method. 6. If you choose to create a new method or edit an existing one, you will be able to edit pump, heater, and detector parameters by clicking on the images of the pump and detector. These parameters include pump flow rate, isocratic or gradient flow type, column heating, and detection wavelength(s). 7. When you have selected your desired method, you can begin setting up the instrument to perform a separation. 60 8. Submerge the vacuum degasser inlet lines in the mobile phase. If your mobile phase will be isocratic, submerge both lines in the same buffer. If you are using a gradient, submerge one line in each of your buffers. 9. Click on the Purge Wizard button in the acquisition bar at the bottom of the window. The Purge Wizard dialog box will open and “Purge Pump(s)” will be checked. Click Next. 10. Prime the pumps. Open the front cover of the binary pump and locate the draw-off valves. 11. Insert a 20 mL Luer-lok syringe into the inlet of a valve and rotate the valve counterclockwise several turns to open. Draw out the plunger of the syringe until several mL of liquid is withdrawn. With the syringe inserted, close the valve, then remove the syringe and empty it. Repeat this step if you see any air bubbles in the inlet tubing for this pump. 12. Repeat with the other draw-off valve. 13. Click Next on the Prime Pump dialog box. 14. The Purge Pump dialog box appears. The pump flow meters will be pre-set at 5 mL/minute. 15. Open the reference valve, located near the draw-off valves for the pumps. 16. Click Next to proceed with purging the pumps. This will last 5 minutes. 17. Click Next when the purge is complete. Close the reference valve and click Finish. 18. Equilibrate the system. Click the Equilibrate button on the acquisition bar. Select the appropriate method from the drop-down list and click Equilibrate. 61 19. Allow the system to equilibrate for 20 minutes, or until the baseline is stable. 20. Click on the Stop Pump button to stop the equilibration. 21. Click on the Inject button to initiate a run. You will be asked for the desired method and information on the sample to be injected. 22. The manual injection dialog box will open. Fill a syringe with the sample and insert into the manual injection port. Inject the sample and using a fluid motion, move the injection port lever clockwise until it stops, then back to the original position. This movement initiates data collection at the detector. 23. The run will proceed until finished. Chromatograms are saved automatically. They can also be annotated and printed at this point. 24. Run another equilibration for at least five minutes to rinse the column. 25. When finished for the day, remove inlet tubes from the mobile phase bottles and close. Shut down pumps, column oven, degasser and detector. 62 SOP HPLC-002 DIONEX HPLC WITH MANUAL INJECTION Revision A, April 21, 2011 Title: Use and Maintenance of Dionex UltiMate 3000 HPLC with manual inject valve 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Dionex UltiMate 3000 HPLC with manual inject valve. 2.0 SUMMARY OF METHOD PART A: INTRODUCTION The Dionex manual inject HPLC instrument consists of several components: an UltiMate 3000 Pump a manual inject valve an UltiMate 3000 Column Compartment, consisting of a column heater and a column an UltiMate 3000 Variable Wavelength Detector (VWD) attached computer with Chromeleon 7 software MATERIALS 1. Dionex UltiMate 3000 HPLC with manual inject valve 2. Mobile phase solutions (HPLC grade) 3. Sample for injection 4. Vacuum filtration assembly and 0.1 um nylon filters 5. 5 mL Luer-lok syringes, 0.2 um cellulose acetate syringe filters PART A: MOBILE PHASE PREPARATION Prior to operating the HPLC, you must filter and degas the mobile phase solutions. Filter the solutions through a 0.1 um nylon filter using a vacuum filtration assembly. This filtration process will also degas the solutions. Be aware that filtering 500 mL of solution can take up to 30 minutes, so provide ample time for this step or prepare in advance. Filter your sample for injection by passing through a 0.2 um cellulose acetate syringe filter. PART B: OPERATION 63 Navigating in Chromeleon 7 The software will display a workstation that consists of several tabs. Each tab represents an HPLC system component (pump, detector, etc), or a system function (audit, startup, queue). The names mean: TCC-3x000: column compartment Ultimate 3000 Home: an overview of the entire system VWD-3x000(RS): variable wavelength detector xPG-3x000(RS,SD): pump Audit: displays an audit trail of every action taken, all alerts the system issues, and all errors that occur. This can be a great way to detect the source of failures. Startup: This is similar the the Home tab, but it also displays the audit trail and shows data from the detector and temperature readings.This is a comprehensive view of what's going on. Queue: this tab will display all the runs that have completed, as well as any scheduled runs. If you do not see these tabs displayed, make sure that the Instruments category bar is highlighted on the lower left side of the screen. Quick Guide 1. Power on HPLC components: pump, column compartment, and detector (switches are on back right of the instrument and can easily be accessed by reaching behind the right side of the components). Power on attached computer and double-click Chromeleon icon. 2. If the Instruments category bar is not highlighted blue at the lower left, click it to enable. You must connect the three instrument components by toggling the Module Connect switch in the software. Click on the column compartment tab (TCC-3x000) and located the Module Connect toggle near the top left of the pane. Click on the left side of the toggle to connect. 64 Give it a few seconds because the toggle does not activate right away. The green light will come on and the module is now connected. 3. Repeat Step 2 with the pump (xPG3x00 tab). You must also start the pump to begin equilibrating. Ensure that the inlet tubing for the channels (A, B, C, and/or D) you'll be using are submerged in filtered, degassed solutions of sufficient volume to purge, equilibrate, and complete the separations you plan to perform. The flow rate should be set less than 1 mL/minute (0.6 mL/minute is a good start). You can set this by typing in the text box under the flow gauge (grey rectangle to the right of the Module Connect toggle). Then click the Continue button (to the left of the Pressure graph) to start the pump. 4. Once the pump has started, you need to purge the pump (this is only necessary when you start the pump at the beginning of the day or when you change eluents). Click the check box next to the box that says "Purge:Off". You will get a warning stating that you should open the purge valve screw. Lift the pump faceplate on the front of the pump to expose the purge valve. The valve screw is brass and in the center of the assembly. Turn counter clockwise about a half turn (if you open too much it will leak). Then click the button on the alert dialog box that says "Execute despite warning". The pump will increase the flow to 3 mL/minute and flush air bubbles out as well as clear any old eluent from the system. Continue until no air bubbles are coming out of the waste line that comes directly from the purge valve. Click the check box next to Purge to slow the pump and then tighten the valve screw (do not tighten the screw first or you could damage the column!). 5. Repeat step 2 with the detector tab 65 (VWD-3x000). You must also ignite the lamp(s) you plan to use; turn on the lamp(s) by clicking the check box next to the name, UV and/or Vis. 6. Once all the components are activated and ready to use, you need to create a sequence. A sequence is a program for the chromatography run(s) you would like to perform in a given session. This can consist of one or more injections using one or more instrument methods. An instrument method is the program for how you would like the instrument components to behave during a chromatography run. Instrument methods can be used again and again, while a sequence can only be run through once and then is saved as a data file. If you have not yet created the instrument method you would like to use for your sequence, skip to step 8 and come back to 7 when you have created a sequence. 7. Create a sequence by clicking Create on the menu bar at the top of the screen. This will start the Sequence Wizard which will help you create a sequence. You will select the number of injections (this can be increased later if desired), the instrument method to be used (if you add injections later you can also use a different method if desired), and then save the sequence with a specific name. 8. Create an instrument method by clicking on the down arrow next to Create on the menu bar at the top of the screen and clicking on Instrument Method. This will start the Method Wizard which will help you create a method. You will select which instrument components to use (the only optional one is the column compartment which controls the temperature of the eluent), and program their parameters. For example, you will program the pumps for the flow rate and eluent composition and the length of time 66 they spend pumping at those settings. 9. When you have the sequence and method(s) how you want them, you are ready to begin a run. Insert a flat tipped syringe containing your sample into the manual inject valve. You should have at least three times the volume of the sample loop in the syringe (standard loop is 20 uL). Ensure that the inlet tubing for your eluent(s) are submerged in the liquid and that you have sufficient volume to complete your run(s). The pump should also be running at the same flow rate as your starting rate for the method you're using (this ensures a smoother baseline). Check the Queue tab for leftover sequences and clear them by clicking remove (on the right of the screen). If you do not do this, the software will run through those before the one you want to do. 10. Open the sequence (if it isn't already active) by activating the Data category bar on the bottom left of the screen and locating your sequence in the menu tree and clicking it. Click the Start button at the top of the pane to begin. The software will perform a Ready Check to ensure that all components are ready to be used; at this point it is common to encounter errors or alerts. Usually errors are accompanied by an indication of how to correct the error. Alerts may require no action but should be noted. See troubleshooting section for a description of common errors and alterts (COMING SOON!). 11. Once the Ready Check is complete you won't see a prompt asking you to inject, but you should inject now (except if there are any errors that need fixing). Load the sample loop by pressing the syringe plunger to release at least three times the volume of the loop. Rotate the valve lever by pressing down on the black plastic lever firmly and leave it in the inject (down) 67 position for about 30 seconds (this time might be longer if the loop is significantly larger, but this is not common). This gives the system time to inject the contents of the loop onto the column. After 30 seconds return to the load (up) position. 12. When you pressed the lever down, the system detected this movement and initated data acquisition at the detector. If you go to the Instrument category bar and go to the detector, home, or startup tabs, you will see a graph with data traces just starting to appear. You can now leave the instrument if needed and it will continue automatically until the current injection has completed its method. If you programmed more than one injection, you will need to press start again and perform the next injection; however, the system will stay on hold between the injections so you don't need to be at the instrument right when the previous segment ends. 13. When a segment of the sequence is complete, you may begin data processing. Double-click the chromatogram thumbnail to the left of the name of the injection to open the data file. A new window opens containing a data processing browser (called the Chromatography Studio). You will see the chromatogram of the selected wavelength trace(s). Below the chromatogram, you can see an empty table. Peak information will appear here when you have chosen a processing method. A processing method is a program telling the software how to recognize peaks and integrate the area under them. In the panel above the chromatogram you will see a button that says Processing Method. Click it and this will move the table down to reveal an Open File icon. Double click this and you will be able to choose from an existing processing method. If none of the existing methods are what you want, 68 go back to the Chromeleon console and use the Processing Method Wizard to create a new method, then go back to the Chromatography Studio and choose it. 14. When you have done your data processing, you may obtain a copy of your data; press the Print Screen key on the keyboard, and then paste this image into Microsoft Paint. You can then save this file as a BMP or JPEG and save to your flash drive. 15. When finished for the day, stop the flow of the pumps by clicking the Stop Flow button in the pump tab, turn off the lamp(s) by unchecking the box next to them, and then power off all three components. 69 SOP INC-001 HERAcell CO2 Incubator Revision A, April 21, 2011 Title: Use and Maintenance of HERAcell 150 CO2 Incubator 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the HERAcell 150 CO2 incubator. 2.0 INTRODUCTION The HERAcell 150 CO2 incubator is a laboratory device for preparing and culturing cell and tissue cultures. The device allows the simulation of the special physiological ambient conditions for these cultures due to the exact control of temperature, CO2 and O2 content, and increased relative humidity. In the ACC Biotech program, this incubator is intended to be used to grow cultured animal cells. 3.0 SUMMARY OF METHOD MATERIALS HERAcell 150 CO2 incubator 50 lb tank of medical grade CO2 with regulator to deliver 0.8-1 bar Sterile tap water, 3 L PART A: SET UP These instructions are for starting the incubator up when it has been off for a while. 1. With the incubator turned off, open the exterior and interior doors. The interior door can be opened by rotating the blue handle. 2. Inspect the interior of the incubator for cleanliness; if it needs cleaning, use a paper towel and 70% ethanol. Allow the ethanol to evaporate before turning on the incubator. 3. Pour 3 L of sterile tap water into the reservoir at the bottom of the interior of the incubator. 4. Close the doors. Make sure the Medical grade CO2 can be ordered from Praxair. 70 incubator is connected to a 50lb tank of medical grade CO2 with a regulator that can deliver 0.8-1 bar (10-20 psi). 5. Open the valve on top of the gas tank (rotate about 3 full turns). Then open the black plastic valve on the side of the regulator (rotate about 3 full turns). Both gauges on the regulator should indicate pressure. Do not move the white valve yet. 6. Turn on the incubator by pressing the on/off switch on the bottom front. Wait a few moments for the incubator to initialize and display the current temperature and CO2. These will most likely be below the set points of 37ºC and 5% CO2, so the yellow indicator lights will be on. 7. Go back to the gas regulator. Look at the gauge on the left; it should register approximately 10 psi. If it is significantly above or below this value, CAREFULLY AND SLOWLY adjust the white valve until the pressure is between 9 and 11 psi. 8. Allow the incubator to equilibrate to the set point temperature and pressure. The relative humidity inside the incubator will increase as the water from the reservoir evaporates into the warm air. No need to change any settings for humidity. 9. After 10 minutes check the temperature and % CO2. If the temperature is too low, give the incubator some more time to equilibrate. Confirm the temperature using a certified thermometer or temperature probe. 10. If the CO2 is too high or low, check the regulator gauge. The right gauge should display several thousand PSI while there is gas in the tank (this cannot be adjusted). The left gauge should be user-adjusted to around 10 psi. IMPORTANT: the CO2 inside the tank is under such high pressure that some of it is liquefied. As gas is consumed and leaves the tank, the liquid amount grows smaller; however, the same pressure will register on the regulator as long as there is liquid CO2 in the tank. What this means is that you will 71 11. When the temperature and % CO2 have reached the set points, the humidity will also have equilibrated to the appropriate level and cells can be safely placed in the incubator. 12. Only open the interior door when you need to add or remove items from the incubator. Try to coordinate these openings with other users to minimize the total number of openings per day. When you open the interior door quickly remove or replace your item and close the door; do not leave the interior door open for any reason if the incubator is on. PART B: MAINTENANCE 1. Be sure to record every opening and closing of the incubator in the equipment log. This helps indicate when a new gas tank will be needed. 2. Check the water level in the water reservoir occasionally. It should come within a few inches of the mark in the bottom of the incubator. If it seems low, add sterile tap water 500 mL at a time. Do not overfill! 3. No regular maintenance of the incubator is required. However, when the incubator will not be used for longer than a week, shut it down to save CO2. Turn the incubator off. Close the main valve on the top of the gas tank and then close the black side valve. If the incubator will not be used for more than a month, remove the water in the reservoir using a vacuum setup and dry the reservoir with paper towels. not observe a decrease in the pressure of gas in the tank UNTIL there is almost none left, then the pressure will rapidly decrease (within the same day or so). Therefore, it is important that the tank is monitored and a spare tank is kept on-site so they can be switched out quickly when the original tank empties. 72 SOP MCP-001 USE OF VWR MICROPIPETTORS P10, P20, P100, P200 AND P1000 Revision A, August 15, 2012 Title: Operation and maintenance of VWR Micropipettors 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for operation and maintenance of VWR P10, P20, P100, P200 and P1000 micropipettors. 2.0 SUMMARY OF METHOD PART A: OPERATION COMMENTS 1. Each student or group should check out a set of micropipettors for the duration of the course. Your instructor will tell you how to record your selection and if you need to validate your set (see Part C). If any micropipettors in your set are damaged, notify your instructor immediately. Check-out will prevent abuse of micropipettors, and ensure that errors in measurement can be tracked to an out-ofcalibration instrument. Note that the most common measuring range for a P10 is 1.0-10.0µL, for P20, 2.020.0 µL, for P100, 10.0-100.0 µL, for P200, 20.0-200.0 µL, for P1000, 100.0-1000.0 µL. Never adjust a micropipettor outside of this range! On P10, P20, P100, and P200 micropipettors, the black digits indicate microliters; the red digits indicate tenths and hundredths of microliters. On P1000 micropipettors, the red digits indicates milliliters and the black digits indicate microliters. The appearance of the minimum and maximum volumes on the analog readout of each size micropipettor are shown in the appendix to this SOP. 2. Select a micropipettor from your set that will measure the volume you need. 3. Set volume using volume adjustment knob. Hold micropipettor in one hand, and with other hand, turn volume adjustment knob counterclockwise so volume indicator is 1/3 revolution above desired setting, then turn slowly clockwise until indicator shows desired volume. If micropipettor dial is past either its high or low limits, or the dial will not rotate, notify your instructor immediately. 4. If you pass the desired setting, turn dial 1/3 revolution higher than desired and reset volume. 5. Attach a new disposable tip to the pipette shaft by pressing shaft into tip while tip is still in box. Press only hard enough to make a positive airtight seal. 6. Press plunger to first stop. This part of the stroke is volume indicator. Always dial down to the volume setting. This prevents mechanical back lash from affecting accuracy. Otherwise, precision and accuracy will be affected. Do not touch the tips. To avoid contamination a fresh tip should be used for each measurement. Never immerse the tip more than a few millimeters below the surface of the fluid. A deeper immersion can cause fluid to cling to outside of tip, delivering more fluid than was desired. 73 7. Holding micropipettor vertically (perpendicular to the bench), immerse the last few millimeters of the disposable tip in the fluid to be measured. Never let the plunger snap up. This can cause air bubbles to fill the tip, destroying accuracy. 8. Release the plunger slowly until it has returned to its original position. 9. Pause for a few seconds to ensure that the full volume of fluid is drawn into the tip. 10. Withdraw the tip from the sample, keeping the micropipettor completely vertical. Always keep the micropipettor in vertical position to ensure that no liquids enter the micropipetter or drip from the disposable tip. 11. Inspect tip to make sure there is no air bubble on the inside and there is no solution on the outside of the tip. 12. If there is an air bubble inside tip, you must pipet this volume back into the sample and measure again, repeating steps 5-10. 13. If there is solution on the outside of the tip, touch tip to the wall of the solution container to remove it. 14. To dispense the sample touch the tip end against the side wall of the receiving vessel and depress the plunger slowly to the first stop, then press the plunger to the second stop, expelling any residual liquid in the tip. Dispense the fluid into the lowest possible place in the receiving vessel. 15. With the plunger fully pressed, withdraw pipette from the vessel carefully, with the tip remaining against the wall of the vessel. 16. Release the plunger to return to the up position. 17. Discard the tip into a waste beaker or trash receptacle by depressing the tip ejector button. Never discard tips into sinks. Always use a waste beaker or trash receptacle. If you measure a hazardous material (chemical or biological), dispose of contaminated tips into a properly labeled 74 PART B: TIP SELECTION 1. Tips must seal properly on the shaft to assure an airtight seal and avoid leaks or poor accuracy. 2. Tips must be soft and flexible so that the shaft is not scratched or worn prematurely. container and store with the hazardous waste. Barrier tips provide the best protection from air contamination, but they do not always fit on the smallest micropipettors. Do not use sterile tips for non-sterile applications. Do not use gel loading tips for non-gel-loading applications. These tips are expensive. 3. Tips must be free from microscopic particles. 4. The tip orifice must be the correct size, and orifice size and geometry must be consistent from tip to tip. 5. Interior and exterior surfaces must be clear, smooth and hydrophobic to avoid retention of liquid. PART C: VALIDATION 1. Micropipettes should be validated at least once a semester. 2. Obtain a printed copy of the blank Micropipettor Validation Log (Appendix). Record the serial numbers and set number of your micropipettor set. Also include the names of people in your group. 3. Locate an analytical balance and calibrate if necessary. 4. Dial a micropipettor to its highest volume. 5. Place a weigh boat on the weighing pan of the balance, close the draft shield, and tare the balance. 6. When balance is stable, measure highest possible volume of purified water with the micropipettor, and dispense into the weigh boat in the balance. 7. Close the draft shield and wait for the balance to stabilize. Record the mass Your instructor will tell you if this process has been assigned to your class. 75 of the water in the validation log. 8. Repeat steps 6 and 7, adding each measurement of water to the weigh boat and recording the total mass of water with each addition. 9. Make ten measurements. Discard the weight boat with water. 10. Repeat steps 4 through 9 with each of the other micropipettors in your set. 11. When complete, enter the data into the Excel file, Micropipettor Validation Log Template. 12. Save and print or email the file to your instructor. Functions programmed in the workbook will automatically calculate the volume delivered in each measurement, and the resulting percent precision. 76 APPENDIX TO SOP MCP-001 USE OF VWR MICROPIPETTORS P10, P20, P100, P200 AND P1000 Updated May 19, 2006 Appearance of Analog Readout at Minimum and Maximum Volumes of Micropipettors P1000 (100-1000 μL) Minimum, 100 μL 0 1 0 Maximum, 1000 μL 0 2 0 Maximum, 1000 μL 0 2 0 Maximum, 200 μL 0 5 0 Maximum, 200 μL 1 0 0 P1000 (200-1000 μL) Minimum, 200 μL 1 0 0 P200 (20-200 μL) Minimum, 20 μL 2 0 0 P200 (50-200 μL) Minimum, 50 μL 2 0 0 P20 (2-20 μL) Minimum, 2 μL 0 2 0 Maximum, 20 μL 2 0 0 P10 (1-10 μL) Minimum, 1 μL 0 1 0 Maximum, 10 μL 1 0 0 77 SOP MIC-001 NIKON TS100 INVERTED MICROSCOPE Revision A, May 27, 2011 Title: Use and Maintenance of Nikon TS100 Inverted Microscope 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Nikon TS100 Inverted Cell Culture Microscope. 2.0 SUMMARY OF METHOD MATERIALS Nikon TS100 inverted microscope Flask or slide with cells Lens papers PART A: INTRODUCTION The Nikon TS100 inverted microscope is intended mainly for use in microscopic observation and in the micromanipulation of living cells and tissue using transmitted and reflected illumination. Our RRC cell culture lab is equipped with six TS100 microscopes. These microscopes have the light source and condenser on top and the objectives on the bottom, allowing enough space on the stage for cell culture plates and flasks. Brightfield illumination is provided by an incandescent bulb. Each condenser contains a phase slider that results in adjustment-free phase contrast images. The TS100 model is very durable and can stay functional as long as 20 years with proper care. PART B: OPERATION—PHASE CONTRAST 1. Turn on the lamp using the green on/off switch at the bottom left. Adjust the brightness of the viewfield with the brightness adjuster (rheostat). 2. Adjust the interpupillary distance. Looking through the eyepieces, adjust the distance between the binocular eyepieces so that the left and right viewfields overlap to form a single The condenser is located just below the lamp housing. There are two inserts in the condenser. The top insert is a colored glass filter; the default is a blue filter that reduces eye strain compared to the unfiltered incandescent light. There are other color filters available that might be used in special situations. 78 image. 3. Set the diopter adjustment ring to the reference position. Turn the ring on each eyepiece to align the 0 line with the reference line. 4. Fully open the aperture diaphragm. Move the aperture diaphragm level on the condenser to the full right to fully open the aperture. 5. Set the specimen in place. Place a flask, dish, or slide on the stage. Adjust its position so that the center of the container comes under the optical path. Be careful that the ring insert in the stage is compatible with the vessel you are viewing (i.e. it will not fall through the stage). Before placing large containers on the stage, be sure that the tip of the objective does not protrude past the stage or it may be damaged. 6. Adjust the diopter. If necessary, adjust the diopter adjustment rings according to the power of your left and right eyes. 7. Observe the specimen. Start with the lowest objective and focus using the coarse adjustment knob, being careful not to hit the specimen with the objective. 8. When focused, rotate the nosepiece to a higher power objective if desired. Focus again, using the fine adjustment knob. Adjust illumination using the rheostat if desired. 9. When finished, lower the nosepiece with the coarse adjustment knob and rotate to the lowest objective. Turn off the microscope and cover. PART C: MAINTENANCE Because the TS100 is equipped with a phase contrast slider, the Kohler illumination does not need to be checked or aligned. In fact, aside from the individual adjustments of oculars and condenser, this microscope requires virtually no adjustments. DO NOT rotate the left and right focus knobs in opposite directions at the same time or continue to rotate the coarse focus past the upper or lower limit of its motion. These actions will damage the microscope. *When rotating the nosepiece, grip by the knob and not by the objective because they can become damaged by twisting or can come loose. 79 Illumination is emitted from the bulb in the lamp housing (black cylinder at very top). The bulb will provide light until it burns out. To change the bulb, squeeze the lamp housing and pull up. Pull out the bulb and insert a new one (handle only with gloves— oils on your hands will shorten the life). One a year, the microscopes should be cleaned professionally. We have a oneyear parts and labor warranty with Nikon, as well as a 5-year parts warranty (for everything but electronics). PART D: TROUBLESHOOTING If there is no illumination, make sure the microscope is switched on, plugged into the wall, and that the power cord is firmly connected to the device (sometimes the cords can come loose). Try turning the rheostat completely up or down. If no illumination occurs, the lamp needs to be reinstalled or replaced. If the viewfield is only partially visible, the nosepiece may not have clicked into place (the objective is not in the optical path). Rotate until it stops with a click. There is a torque adjustment wheel on the coarse/fine adjustment knob that can be used to tighten or loosen the coarse and fine adjustment. If this is too loose, the stage can slide down with gravity. Try to keep the torque at a moderate level and do not over-adjust it. 80 SOP MIC-002 NIKON 55i FLUORESCENCE MICROSCOPE Revision A, April 21, 2011 Title: Use and Maintenance of Nikon Fluorescence Microscope 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Nikon Eclipse 55i Microscope with Fluorescence Accessory. 2.0 SUMMARY OF METHOD MATERIALS Nikon 55i upright light microscope Fluorescence light source and power supply Camera and control center/view screen Fluorescent sample on slide for viewing Immersion oil (if using 100x) Lens papers PART A: INTRODUCTION The Nikon fluorescence microscope can be used to observe fluorescently labeled cells or intracellular structures. It consists of a basic Nikon 55i upright light microscope, fluorescent light source and power supply, CCD camera, and a camera control center/view screen. The CCD camera takes high-resolution images and displays them on a view screen that is also the camera control center. The control center has firmware that can be used to capture, save and annotate the images. There are 20x, 40x, and 100x objectives installed; oil immersion can be used at the 100x power. PART B: OPERATION Fluorescence Illumination 1. Turn on the fluorescence power supply using the toggle switch. Turn on the microscope and camera control center. *It is not necessary to warm up the metal halide lamp before capturing images (this only matters if you are quantitating fluorescence intensity and we cannot do that with the software we have). When using fluorescence, turn the power supply on before the microscope and camera control, because the surge of power from the metal halide lamp could possible damage the optics of the camera. For the same reason, turn the power supply off last. After the metal halide lamp has been turned off, there is a 20-minute auto delay before it can be turned back on. If you try to turn it on 81 2. Turn off Brightfield illumination by turning the rheostat down until no light shines through the condenser. The Brightfield condenser must be turned off in order to see fluorescent images. Additionally, you should also cover the condenser with something dark and non-reflective (like dark matte fabric or paper) because reflected ambient light can also saturate the pixels of the camera and drown your fluorescent image out. You should also turn off overhead lights, especially fluorescent lights, because they will affect the quality of the image you see. 3. Select the filter cube appropriate for the fluorophore you are looking at in your sample. We currently have three filter cubes: for DAPI, FITC, and TRITC stains. The FITC will also work to visualize GFP. The DAPI can be used for Hoescht mycoplasma stain. These are all long-pass filters, which will allow you to visualize any fluorescence at a wavelength above the emission filter wavelength. So, you may see other colors of fluorescence besides the one you are looking for. There are also band-pass filters, which allow just a narrow range of wavelength light through. These are necessary when quantitating fluorescence intensity because you should only count emission of a single color. Filter positions: 1 empty 2 DAPI (UV) 3 FITC (GFP) 4 Rhodamine (TRITC) 4. Place your sample slide on the stage between the clips until square. Rotate the nosepiece until the objective you want to use is over the sample (should be 20X or 40X at this point). You should see some type of image on the view screen. You do not have to use the oculars to view the sample, as the image should appear in the view screen just as it does in the oculars (with the exception that the oculars can be during the delay, the light will flicker. Make sure to always turn off the light source when not in use. The rheostat is a small knob located on the right side of the microscope body. *The light passes through one of several filter cubes, which will only allow one wavelength of light through to the sample. This excites molecules in the sample, which emit a higher wavelength light; this light passes through another filter on the cube and then travels to the camera and your eye. The filter cubes rotate around in the nosepiece via a knob on the right side. The number on top of the knob corresponds to the active filter. There is also a filter lock toggle switch on the front of the nosepiece. When activated, you cannot switch the filters. Keep this out at all times so you can switch the filters. *When rotating the nosepiece, grip by the knob and not by the objective because they can become damaged by twisting or can come loose. The oculars (eyepieces) are individually adjustable and also telescope out and 82 adjusted for individual eye differences). 5. If the image appears too bright on the view screen, you can engage one or more of the neutral density filters to dim it. 6. Using the coarse adjustment knob, adjust the stage up or down to focus on the sample until you achieve a good image. Then use the fine adjustment knob to fine-tune the focus. If desired, rotate the nosepiece to select a higher magnification and fine adjust again. 7. When the image you have on the view screen is satisfactory, you may capture images Capturing Images The CCD camera will capture what you see in the ocular and display it on the view screen/control center. 1. To capture an image, simply press the Capture button on the view screen. Under the CAM menu there are many settings that can be changed to obtain a better image, including contrast, color, exposure time, etc. There are some presets that are comparable to scene settings on your digital camera. You can also save your own settings and recall them later. The control center has firmware that can be used to annotate and customize images. There are also annotation tools that can be used to draw lines, circles, labels, and other features, and you can also calculate distances between structures or the area of a region. These can be found under the TOOL menu. Use a USB storage device to save your images. You can insert the device directly into the control center. There is also an 8GB memory card that can be used if you forget to bring a USB storage device. The files are saved with the file name stem down for ergonomics. *There are a series of neutral density (ND) filters on the microscope body and on the lamp power supply. These will dim the intensity of the light without changing the color. This can be helpful to prevent photobleaching of a sample from overintense light. The number on the filter (2, 4, 8, 16) corresponds to the factor by which the light is reduced (by half, ¼, etc). *There is a torque adjustment wheel on the coarse/fine adjustment knob that can be used to tighten or loosen the coarse and fine adjustment. If this is too loose, the stage can slide down with gravity. Try to keep the torque at a moderate level and do not over-adjust it. * There is a knob on the front of the microscope that opens and closes the shutter for fluorescence illumination. You can close this if you want to stop illuminating temporarily, but do not want to turn off the lamp (because of the 20minute delay). You might want to use this if you have to walk away from your microscope for a few minutes, but want to avoid photobleaching the sample on your slide. 83 “IMGBOX” and then with the time and date. You can change the file name stem but the time and date will still be included. This menu can be reached from the SETUP menu on the main screen. You will have the option to save files as JPEG, BMP, or TIF. These files are the same format as digital camera files so they should open with no problem on virtually any operating system. There is no attached printer, so you will need to print on a regular office printer. The VIEW menu allows you to recall saved images. Under the REC menu, you can command the firmware to take a series of timelapse photos (not at video speed, but similar to a video). PART C: MAINTENANCE Brightfield illumination is provided by an LED light that produces very little heat and never needs replacing. LED light also causes less eye strain than traditional incandescent light. The condenser does not have phase contrast. The fluorescent lamp is housed inside the power supply. It is a metal halide lamp that lasts about 2,000 hours. There is a counter on the power supply that records the hours that you can reset when you replace the bulb; it should last around 2000 hours. A replacement lamp costs $695 and it is very easy to replace, but a technician from Nikon can do this if you buy the lamp. PART D: TROUBLESHOOTING 1. After the metal halide lamp has been turned off, there is a 20-minute auto delay before it can be turned back on. If you try to turn it on during the delay, the light will flicker. 2. On the bottom right of the microscope 84 body there are two rods that are used as an on-off switch for the color balance filter. The top rod in is the “on” position. Activate this if you perceive the images as being too blue when using Brightfield, which can happen because of the LED illumination. 3. On the bottom right side of the microscope body there is a small white button that says PRE (for “preset”). This is the autophoto button, which will freeze all light settings. This can be helpful if you want to take several images with the same settings and don’t want to accidentally change anything. If the light controls will not change even when you move the controls, make sure this button is not pressed in. 4. The light from the lamp travels from the power supply through a thick fiber cable to the microscope body. It is very important to not bend or crush this fiber because it will dim the intensity of your illumination. If you are having illumination problems, one of the best quick fixes is to replace this cable. 5. If there is a discrepancy between the images you see in the ocular and on the view screen, first adjust the ocular to account for your eyesight. If they still don’t agree, you can adjust the parfocal adjustment control which is a wheel under the camera. 6. The camera on/off slider is located on the top right of the microscope body. When in the on position, this causes the image to be split between the eyepieces and the camera. It must be on when taking photos, but need not be on when simply observing specimens. If your signal is dim while observing, you can try turning this off until you need to take a photo. 85 SOP MPR-002 USE AND MAINTENANCE OF BIOTEK SYNERGY 4 MICROPLATE READER Revision A, June 8, 2011 Title: Use and Maintenance of Synergy 4 Microplate Reader 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for use and maintenance of the BioTek Synergy 4 Multimode Microplate Reader. 2.0 INTRODUCTION The Synergy 4 multimode microplate reader is capable of UV and visible absorbance readings, as well as bottom- and top-reading fluorescence. It can only be operated using the Gen 5 software on an attached computer. The Gen 5 software can be installed on up to five computers. Computers must have Windows XP or Vista, Microsoft Excel for exporting data, and at least 1 GB of RAM. The reader contains both a tungsten lamp with a filter wheel and a xenon flash lamp with a monochromator. Continuous wavelength tuning using the monochromator is available for both absorbance and fluorescence modes. There is a limited selection of filters provided; however, additional filters can be ordered. The advantage of filters is increased sensitivity and specificity. The tungsten lamp requires 3 minutes to warm up. The xenon flash lamp does not require a warm-up period. The reader can be programmed to incubate at temperatures from ambient to 42ºC. The incubator requires 15 minutes to warm up. It can also be programmed to shake plates before reading. In absorbance mode, the reader can measure up to 6 wavelengths at a time. It can also obtain an absorbance spectrum of each well. Greiner brand 96-well plates are recommended. For UV absorbance, use clear plates coated with a substance to make them transparent to UV. For visible absorbance, any transparent plastic will work. For top-reading fluorescence, use solid black plates; for bottom-reading fluorescence, use solid black plates with clear bottoms. Never use lids on plates because they will make the plate too tall and the lid will damage the optics. Avoid using sealing film or tape on plates because the optics will be located very close to the top of the plate. 3.0 SUMMARY OF METHOD COMMENTS PART A: OPERATION USING QUICK READ 1. Turn on lamp power supply (gold box connected to power cord) and instrument (switch is on front panel). The instrument will initialize; some noises are normal. 2. Connect laptop computer to the instrument via a USB cable and start computer. Open the Gen 5 software. 3. At the Welcome screen, click “Read a Plate.” 86 4. Design a method by adding steps (such as Read, Shake, Delay, Temperature, etc). You must add at least one Read step, but the other steps are optional. 5. When you add a Read step, you will be given the following options: a. Detection Method (choose Absorbance) b. Read Type (choose Endpoint) c. Read Speed (choose Normal) 6. Choose the number of wavelengths and enter them in the drop-down menu(s). Click OK when finished with this Read step. 7. Enter Delay, Temperature Set, Shake or more Read steps if desired. When you have finished designing your procedure, you may click Validate to make sure the order of your steps is logical (this is only useful when you have entered more than one step). When designing a protocol, you will be able to choose your plate manufacturer from a drop-down menu. The design of each plate will affect the protocol slightly (usually, the plate height affects the distance of the optics from the plate). Therefore, it is important to choose the correct plate from this list. If it is not on the list, choose Generic. If you have full wells (300 uL), do not use the fast shake option because your sample may splash out of the plate. Plates should shake for 3-5 seconds, followed by a 2second delay before reading. If you do not turn on the Tungsten lamp, the Xenon flash lamp will be used. The Xenon lamp does not need to warm up before use. 8. Click OK when finished designing your procedure. Enter a plate ID on the next menu and add comments if desired. 9. Click Read. The Save As dialog box will open; give your data set a unique name and click Save. 10. The sample tray will open. Insert your plate with well A1 oriented towards the upper left corner. Click OK, the tray will close, and the reader will scan the plate. 11. The tray will open when the procedure is finished. Remove the plate and manually close the tray by pressing the black eject button on the front panel of the instrument. 12. A window with a schematic of the plate containing data will appear. You may need to choose the wavelength in the Data drop-down menu to see the data. No more than 350 µL should ever be added to a microplate for use in this instrument, due to the risk of spills. 87 13. To export data to Excel, click the Excel icon next to the Data drop-down menu. 14. To read another plate with the same procedure, click the Add Plate icon on the toolbar. Follow the above directions, starting with entering a plate ID. PART B: MAINTENANCE 1. Routine maintenance of the instrument should include cleaning the plate carrier to remove sample residue (an alcohol prep pad can be used) and blowing dust from the filters using canned air. This should be performed about once every six months. 2. To keep the reader in optimal condition, keep the plate carrier clean and free from spills, dust, and debris. Never leave plates in the reader after reading them, because water will evaporate and condense inside the instrument, ruining the optics. 3. About once a month, run a System Test to confirm that the reader is working properly. Choose System > Diagnostics > Run System Test. The reader will run a series of diagnostics to evaluate performance and will pass or fail. If the reader fails the test, contact Technical Support with the error code provided. Most failures are lamp-related. 88 SOP PCR-001 MyiQ iCycler Real-time PCR System Revision A, June 8, 2011 Title: Use and Maintenance of MyiQ iCycler Real-time PCR System 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for use and maintenance of the iCycler thermal cycler with the MyiQ Single-color Real-time PCR Detection System. 2.0 SUMMARY OF METHOD MATERIALS iCycler with MyiQ optical module Computer with iQ5 software PART A: SETUP 1. Check that the base unit (thermal cycler) is connected to a USB port on the computer. The base unit should also be connected to the optical module (upper unit) by a serial cable. Both units should be plugged in to power outlets. 2. Turn on the base unit using its power switch; turn on the MyiQ optical module using its power switch. 3. Double-click the Bio-Rad iQ5 icon on the computer desktop. 4. Perform background calibrations (see Appendix 1). 5. Choose the Workshop tab; highlight the Setup tab, then the Protocol tab. 6. To select an existing protocol, click the protocol name. The selected protocol appears in the Selected Protocol window. 7. Edit a selected protocol by clicking Edit in the Selected Protocol window. 8. Create a protocol from a protocol template by clicking Create New in the Selected Protocol window. 89 9. Edit the protocol by performing one or more of the following five tasks: a. Edit the Dwell Time and Setpoint temperature. Click in the Dwell Time or Setpoint cell, and then enter the Dwell Time or Setpoint temperature. To enter 10 seconds, type 0 followed by : then 10 (that is, as the time appears in the spreadsheet). Alternatively, 10 seconds can be entered as 0.10. b. Acquire data. Click in the Data Acquisition column, and then click Real-Time at the step you want to collect real-time data. Click Melt Curve if data from a melt curve is required. c. Insert cycles and steps. Insert a cycle by clicking in the Insert column within the cycle row. Cycles have a blue background. iQ5 software inserts the new cycle below the current cycle. Insert a step by clicking in the Insert column within a step row. Steps have a white background. iQ5 software inserts the new step below the current cycle. d. Delete cycles and steps. Delete a cycle by clicking in the Delete column within a cycle row. Cycles are indicated with a blue background. Delete a step by clicking in the Delete column within a step row. Steps are indicated with a white background. e. Save the protocol. Click Save & Exit Protocol Editing. Type the name of the protocol in the Save As dialog box, and then click Save. 10. Create a plate setup by clicking the Create New button under “Selected Plate setup.” 11. Enter a file name, and enter any notes about the plate setup in the Notes box. 12. Enter or edit the sample volume, seal type, and vessel type. 13. Enter or edit a name for the experiment. 90 14. Click a sample type icon. 15. Select the type of replicate loading desired. 16. Click on FAM under “Fluorophore.” 17. Click or drag across the plate to define wells with the selected fluorophore (FAM) and sample type. 18. Continue defining the remaining wells by changing to the other sample type icons. 19. To delete a previously defined well, click the Erase Well(s) icon and then click the well. 20. To delete the selected fluorophore from a previously defined well, click the Erase Well(s) by Selected Fluorophore icon and then click the well. 21. If there are standards in the first dye layer, click Dilution Series and calculate the concentrations of the standards, or type the concentrations in the spreadsheet manually. 22. Click Save & Exit Plate Setup. Give the plate setup file a new name or overwrite the current version of the file. The software adds the extension .pts automatically. PART B: OPERATION 1. In the Protocol tab, click Run. The Initiate Run screen will appear. 2. Select Use Persistent Well Factors. 3. Click Begin Run. 4. Name the file in the Save Optical Data File dialog box and click OK. 5. Run will initiate and the Monitor Run window will open. 6. Thermal cycler will hold amplicons at 4ºC overnight if necessary. 91 PART C: TROUBLESHOOTING PROBLEM: After opening the software, an error message appears: “Cannot detect camera or thermal cycler.” SOLUTION: Check that the serial cable is connected between the base unit and optical module. Check that the base unit is connected to a USB port on the computer. You may have opened the software before the base unit finished initialization. Turn off base unit and optical module, close the software, turn base unit and optical module on and allow them to initialize. Then open the softare. 92 Appendix 1: Calibration of MyiQ Detection System NOTE: The detection system needs to be calibrated once a month, or whenever users change sample volume or vessel type. Therefore, this procedure should generally be performed each time the detection system is used. MATERIALS 1. iCycler with MyiQ detection system 2. External Well Factor Solution (Bio-Rad) PART A: MASK ALIGNMENT 1. Determine the sample volume and type of vessel you will be using for your experiment. 2. Prepare enough 1X External Well Factor solution to fill 96 wells with your sample volume. Dilute the 10X EWFS to 1X with water. 3. Pipette the sample volume into the correct vessel(s) and seal with the appropriate cap or tape. Load vessel(s) into the thermal cycler sample compartment. 4. With the thermal cycler and detection system on and the software open, click the Calibration tab. 5. Click on the Mask tab (it will be highlighted in green). 6. Click on Home, then highlight the Filter Position 2 option. 7. Click on Take an Exposure, then click on Show Mask. 8. If any pink pixels are present in the mask, reduce the exposure time and retake an exposure. Keep reducing the exposure time and retaking the exposure until no pink pixels are present. 9. Click Optimize Mask, then click Save Mask. For example, this could be 50 µL sample volume in a 96-well plate with sealing tape, or it could be 25 µL sample volume in strip tubes with flat caps. Prepare a volume in excess of what is actually needed so you do not run out. A multichannel pipettor is a useful tool for filling the plate or tubes. Wear gloves when handling optically clear plastics to avoid leaving fingerprints. 93 10. Remove well factor plate, tubes or strips and save for Part C. PART B: BACKGROUND CALIBRATION 1. Load an empty sealed plate, or capped strips or tubes into the sample compartment to fill it completely. 2. Select the Background tab. 3. Select a seal type and a vessel type, and click on Collect Background Readings. The software will launch a short run that will last about 10 minutes. 4. When the background run is complete, remove the empty vessel(s) and save in a plastic bag until this calibration needs to be repeated. PART C: WELL FACTOR CALIBRATION 1. Place the external well factor plate or tubes in the sample compartment. 2. Select the Well Factor tab. 3. Select a sample volume, a seal type, and a vessel type. 4. Click Collect Persistent Well Factors. The software will launch a short run that will last about 10 minutes. 5. When the PWF run is complete, remove the vessel(s) and save or discard. The instrument is now ready for quantitative PCR. The 1X well factor solution has a shelf life of one month. 10X solution keeps in the -20 freezer for a year. 94 SOP PCR-004 APPLIED BIOSYSTEMS 2720 THERMAL CYCLER Revision A, February 22, 2011 Title: Operation of Applied Biosystems 2720 Thermal Cycler 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the AB 2720 thermal cycler. 2.0 SUMMARY OF METHOD MATERIALS AB 2720 thermal cycler PCR reaction tubes, strips, or 96-well plate PART A: OPERATION 1. Plug in the thermal cycler and turn it on. You will see a Home screen with the time, date, and temperature at the top and RUN, CREATE, EDIT, UTIL, and USER at the bottom. 2. Use the F1-F5 softkeys to select one of the options at the bottom of the screen: a. F1 to RUN an existing protocol b. F2 to CREATE a new protocol c. F3 to EDIT and existing protocol 3. Run a protocol that is already saved. Choose the protocol you want with the arrow keys and hit the softkey under VIEW to check the program. If the program is correct, hit the softkey under START to begin. You must enter the volume of your reaction mixture before the program will begin. At this step, open the lid and insert your reaction tubes, strips, or plate into the sample block, close the lid, and proceed. 4. Create a new protocol. Use the arrow keys to move through the screen and use the number keys to enter the appropriate times and temperatures. When you are finished you may SAVE or IMPORTANT: this instrument will only hold 0.2 mL sample tubes. If your reaction is in a 0.5 mL tube, you MUST transfer it to a 0.2 mL tube. 95 START. 5. Edit an existing protocol. Choose the protocol you want with the arrow keys and hit the softkey under VIEW to edit. Make the necessary changes and SAVE. 6. When you have started a protocol the instrument will warm up to the start temp and then begin the program. There is no pause before the program starts, as there is with some other instruments, so make sure your samples are in the block before starting. 7. Every protocol has an automatic hold at 4 degrees at the end of the protocol, so your samples will be chilled after the run. Still, you should attempt to remove them as soon as possible after the run and freeze them. 96 SOP PCR-005 CFX96 Real-time qPCR System Revision B, April 11, 2011 Title: Use and Maintenance of CFX96 Real-time qPCR System 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Bio-Rad CFX96 realtime qPCR system. 2.0 INTRODUCTION The CFX96 is the newest qPCR instrument from Bio-Rad. It uses LED technology to excite up to 5 fluorophores in each well to monitor and quantify PCR products. The major benefit of this instrument is that it allows the user to quantify the PCR products below cycle 30 (above which the results are considered unreliable). A great feature of the CFX96 is that the user does not need to do any time-consuming calibration steps or plate setups before their PCR run. There are internal diagnostic procedures that make calibration unnecessary. Reading an entire 96-well plate takes only 3 seconds if using one channel (12 seconds if using all five channels). The instrument automatically reads all wells. The instrument is controlled by CFX Manager software, but it can be used without the software as well. The software can be operated (protocol and plate design and data analysis) without the instrument connected. The software works on Windows XP and above. Data can be exported to an HTML or Excel file on the computer or directly onto a USB drive if using the instrument without a computer. 3.0 SUMMARY OF METHOD MATERIALS 1. CFX96 real-time qPCR system 2. Computer with CFX Manager software 3. Optically clear plates for PCR; sealing film 4. qPCR master mix reagent PART A: OPERATION To perform a qPCR run, follow these steps: Design a PCR experiment with thermal program, plate setup, data collection, and a melt curve Pipette reaction mixtures into a plate Load plate into the sample block and start the experiment 1. Open the CFX Manager software on 97 the attached computer. The Startup Wizard will appear. You can choose to create a new experiment, repeat an existing one, or open data files. Choose Create a new experiment. Alternatively, instead of using the Wizard you can select this option from the File menu. 2. In the Protocol tab, you can select an existing protocol and use or edit it, or create a new one. If you have chosen to edit or create a new protocol, you will open the Protocol Editor. With this tool, you can edit the temperature, dwell time, and number of repeats of each step of a protocol. Click on the value you want to change in either the graphic or in the text view and enter the desired value. You can also insert or delete steps using the buttons to the left. 3. The protocol template that opens when you create a new protocol already includes a plate read step. To add a plate read, click the step you want, and then click the Add Plate Read to Step button. 4. For a melt curve, click the Insert Melt Curve button. Edit the melt temperature range or increment time. 5. In the Plate tab, you can select an existing plate setup and use or edit it, or create a new one. The plate file contains a description of the contents of each well, the scan mode, and the plate type. To run a real-time experiment, the plate setup must include at least one well with a sample type and fluorophore, and you must select a scan mode and a plate size and type. Everything else about the plate setup can be altered before, during, or after the run. 6. The Plate Editor Window displays the plate layout and provides tools to edit it. Go to Settings in the Menu Bar to set the plate size (number of wells) and The Experiment Setup Window includes three tabs: Protocol, Plate, and Start Run. The quantitation (read) step should always be after the extension step of PCR. The melt curve portion of the program has automatic settings that increase the temp in 0.5 degree increments from the annealing temperature up to 95 degrees. The instrument always reads all wells, so there is no need to design a plate layout before the run. You can also scan in All Channels if you are using more than one fluorophore, or a different one than SYBR/FAM. 98 plate type (color). Choose 96-well and clear. 7. In the Scan Mode drop menu, choose SYBR/FAM only. 8. To label wells, first select them by left clicking on the graphic. You can click, hold and drag to select multiple wells. 9. Load the Sample Type first. Select a Sample Type from the drop menu, choosing from the following: Unknown: an experimental sample you don’t know the concentration of Standard: a sample of known concentration NTC: no (DNA) template control Positive control: control reaction with target DNA added Negative control: control reaction without the target DNA added (may have other DNA template added) 10. Click a Load box to add a fluorophore to the selected wells. You can also specify a target for one or more fluorophores, but this is only necessary when multiplexing or if the data will be used later for a gene study. 11. Sample Name can be skipped unless you are compiling the data for a gene study. 12. To enter a series of standards to generate a standard curve for quantitation, you need to select the wells that contain the series and add the fluorophore and sample type (standard). Then load them as replicates by checking the load box next to Replicates. Click the Replicate Series button and an editor will open. Leave the Replicate Number and Starting Replicate # at 1. This will number the series starting with Std-1. Choose horizontal or vertical (depending on if you did the standards in a row or a column). Click 99 Apply. 13. Click the Load check box next to Concentration and enter the concentration of the first standard. Click the Dilutions Series button. Enter all the information, including the starting and ending replicate numbers, the dilution factor, and whether the concentrations are going up or down as the replicate numbers go up. Click Apply. 14. Repeat for any other standard series you have on the plate, but change the starting replicate number to distinguish the series from each other. Do NOT force the lid back—it operates via a motor and you need only to touch the button to make it move back and forth. Do NOT block the lid from opening or closing. Make sure there is clearance behind and above the instrument for the lid to open, and when you are closing the lid, make sure there is nothing between the lid and the base that would prevent it from closing. Our sample block is designed for plates with 0.2 mL wells only. Optically clear plates and sealing film MUST be used in this instrument. Do NOT use strips and caps because the vessels need to be low-profile or the optics will not function correctly. 15. After editing or creating a new plate, save it and move to the Start Run tab. In the Start Run tab, you can set the run settings and begin the run. 16. The Start Run tab lists the protocol and plate setup you are going to use and has a Start Run button that you click when you are ready to run. When you are ready to load your plate into the sample block, press the Open Lid button on the software’s Start Run tab, or press the lid button on the front of the instrument to open. Be patient, as the lid opens slowly at first. 17. Place the sealed plate on the sample block with well A1 in the upper left of the block. Click the Open Lid button in the software or press the lid button on the front of the instrument to close the lid. 18. With the lid closed, click the Start Run button in the Start Run tab. You will be prompted to save the name of the data file and then the Run Details Window will open. Here, you can monitor the progress of the run. The Run Details Window has three tabs: Run Status, Real-time Status, and Time Status. The Run Status tab shows the status of the protocol and DO NOT leave the unit holding at 4 degrees overnight, since condensation can form on the optics and damage them. Remove amplicons promptly from the unit after the run is over. 100 allows you to open the lid, pause or stop the run, and skip or repeat steps. The Real-time Status Tab shows the real-time fluorescence data as they are collected. The Time Status tab shows a full-screen countdown timer for the protocol. 19. When the run has finished, open the lid, remove the plate, and close the lid. Turn off the instrument and close the software. PART B: DATA ANALYSIS 1. Open a data file by going to File and Open Data File. The Data Analysis window will open. Click the Quantitation tab to see an amplification chart and standard curve(s). Click on wells in the plate graphic to select or deselect wells to display data for. 2. The software automatically subtracts the baseline from well data. You can change this manually by going to Settings and Baseline Threshold. 3. The cycle threshold line is calculated automatically by the software, but you can change this by dragging it up or down using the mouse. 4. To create a report, click the Report icon on the toolbar of the Data Analysis window. Click checkboxes in the report options list to include specific information. Check the preview on the right side of the screen before saving or printing. 5. To export data files into Excel, right click on the table and selecte Export. PART C: TROUBLESHOOTING Experiment Design If the Experiment Setup Window does not show a Plate tab, then the Protocol You can view your melting data in two formats: melting curve (RFU vs temperature) and melt peak (the first derivative of the melt curve). The overall negative slope of melt curves is due to the buffering capacity of the mixture changing with temperature. You’re interested in whatever melts above 80 degrees C. A rule of thumb is that the melting temperature increases 2 degrees C per base pair. 101 selected does not have a plate read step. Add a plate read step to the protocol and the Plate tab will appear. PART D: MAINTENANCE The LEDs have a 20-year lifetime so should almost never have to be replaced. If you change the plate type (from clear to white) you will be asked to calibrate the system using that plate and the selected fluorophore. 102 SOP PHM-002 OPERATION AND MAINTENANCE OF ACCUMET MODEL AB15 pH METER Revision A, June 8, 2011 Title: Operation and standardization of AB15 pH meter 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for the proper operation and standardization of the AB15 pH meter. 2.0 SUMMARY OF METHOD MATERIALS COMMENTS AB15 pH meter pH 4, 7, and 10 standard buffer solutions (if calibrating) 50-mL beakers pH probe with electrode Deionized water in wash bottle Waste beaker (250-500 ml) Labeling tape and sharpie PART A: STANDARDIZATION 1. Turn on pH meter and allow it to warm up for at least 5 minutes. The probe must be supported by the holder. 2. Select two standards that bracket the expected pH of the solution to be measured. If basic, use standards of pH 7 and 10. If acidic, use standards of pH 4 and 7. If the expected pH is unknown, use standards of pH 4, 7, and 10. The tip of the probe should be immersed in a storage solution but not touching the bottom of the vessel (this can damage the probe). Do not keep a pH probe out of a solution for longer than a minute; it may damage the probe. Do not turn probe upside down. 3. Label 50-mL beakers with the pH of the selected calibration standards. Pour 2535 ml of standard buffers into the appropriately labeled beakers. 4. Check that the pH meter is in pH mode (on the top left of screen); if not, press and release the mode key until the meter is in pH mode. 5. Completely rinse the probe with deionized water (over the waste collection beaker) and immerse the electrode 1 cm in a buffer. Wait for the The tip of the probe should not be touching any surface. 103 reading to stabilize, and if it is greater than ±0.03 pH units from the true pH value, then you must standardize the meter. If not, proceed to PART B. 6. Press the setup key twice and then the enter key to clear the existing calibration. 7. Press the std key to access Standardization mode. The selected buffer group will appear (2, 4, 7, 10, 12). 8. Press std again to initiate standardization. The meter will recognize the buffer and flash the pH value on the screen. When the stable icon appears, proceed to step 9. 9. Completely rinse the probe with deionized water (over the waste collection beaker) and immerse the electrode 1 cm in the other buffer. 10. Repeat steps 7, 8, and 9 with this buffer and a third if necessary. 11. Completely rinse the probe with deionized water (over the waste collection beaker), and immerse the tip of the electrode into a storage solution in a 50 mL Erlenmeyer flask. PART B: OPERATION 1. Check that the meter is in pH mode (on the top left of the display) and if it is not, press and release the MODE key until pH appears on the display. 2. Completely rinse the probe with deionized water (over the waste collection beaker) and immerse the electrode 1 cm in the sample. Wait for the reading to stabilize. 3. Record the pH measurement and remove the probe from the sample. Rinse thoroughly with deionized water (over the waste collection beaker) and replace in the storage solution. When the meter accepts the second buffer, it will display the percent slope associated with the electrode’s performance prior to returning to the measure mode. If the electrode is with in the range of 90-102%, the GOOD ELECTRODE message will appear. If the electrode is outside this range, the meter will display ELECTRODE ERROR message and will not return to the measure screen until the user presses the enter key. Add any comments, such as an error message on the meter, low fill solution in the probe, or low or contaminated probe storage solution, to the appropriate log sheet found in the equipment log book. The meter will permit the user to use an electrode outside the recommended range, but the ERROR message will still show. Try recalibration (steps 6-10), if you get an ELECTRODE ERROR message. If you get this error message again after the second calibration, report the electrode failure immediately to your supervisor and enter this on the instrument log sheet. 104 105 APPENDIX TO SOP PHM-002 pH PROBES FOR THE ACCUMET MODEL AB15 pH METER The selection of the appropriate pH electrode (probe) to use with a pH meter can have an impact on the quality of measurements and on the life of the equipment. As of May 14, 2010, we are using Fisher Scientific accumet Liquid-Filled Mercury-Free pH/ATC Epoxy Body Combination Electrodes (catalog# 13-620-631) with our pH meters. These have a double junction with an Ag/AgCl electrode. They can be filled with saturated KCl solution (catalog# SP138-500). These electrodes are mercury-free, refillable, and are safe to use with Tris. You may also see VWR SympHony refillable calomel electrodes (catalog# 14002-772). They can be filled with calomel fill solution (catalog# 34108-024). 106 SOP RCV-001 RECEIVING MATERIALS Revision A, April 21, 2011 Title: Receiving and Storing Materials 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for receiving packages and processing the materials received. 2.0 SUMMARY OF METHOD COMMENTS PART A: RECEIVING 1. Locate the packing slip(s). 2. Find a department name, contact name, or account code on the slip. Ensure that this data corresponds to the Biotechnology department. Our account code is “55506”. The full account number is a 16-digit number and “55506” (or another account code) will appear after “10-1-.” 3. If the shipment has been misdirected, determine the correct recipient of it and redirect. Look for a contact name or department on the packing slip, or call the warehouse at 223-1042 to report. 4. Confirm that the packing slip and actual inventory of shipment are in agreement. Were all items received and in the correct quantity? If any items were reported shipped on the packing slip, but were not in the shipment, call the warehouse at 223-1042 to investigate. 5. Open parcels and check for damaged or missing items. If items are damaged, non-functioning, or incorrect, call the vendor immediately to arrange for exchange or replacement. Be sure to save all packing materials in order to send the items back. 6. Locate the manual or product insert for each product and follow any unpacking, assembly, or storage directions. 7. Manuals and product inserts can be stored with the product (if feasible), or in the file cabinet under the name of the vendor or manufacturer. 8. If the item is a capital asset (generally, over $500), write down the following information: manufacturer, model, serial number, location (campus, building and room number). Send this to the ACC Asset Management 107 department. 9. Perishable items should be stored at the recommended temperatures. Before storing, note the month and year of receipt on the label of the item. 10. Non-perishable items can be stored in the documented storage location (see Equipment Locator). 11. Record the new item on the refrigerator or freezer log, if applicable. Be sure to note the date it arrived. 108 SOP SOL-001 LABELING SOLUTIONS Revision A, January 17, 2008 Title: Labeling Prepared Solutions 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for labeling solutions prepared by students, faculty and staff. 2.0 SUMMARY OF METHOD COMMENTS 1. Obtain a solution prep form from the instructor or from the lab form files. 2. Complete any calculations and enter them at the bottom of the solution prep form (or reference a page in your lab protocol). 3. Obtain all chemicals, glassware, and equipment needed to prepare the solution and assemble so they are all within reach. 4. Make a label for the vessel in which you will prepare the solution; use whatever name you would usually call the solution (for example, “1X TAE buffer”). Leave plenty of room for information that will be added later. Affix the label to the vessel before adding any chemical. Any solution or chemical must be labeled with its identity if it is removed from its primary container (i.e. the stock bottle). 5. Think of a unique ID number (control number) for the solution. The format of control numbers in our labs should follow the following example: The purpose of control numbers is for any person working in the lab to discriminate between any two solutions, regardless of how similar they are, by the control number. TAE-EG-041106 The first three letters are an abbreviation of the common name of the solution (in this example, TrisAcetate-EDTA buffer). The second two letters are the initials of the person (or one of the people) who prepared the solution (Evelyn Goss). The last six digits are the date (always YYMMDD; in this example, November 6, 2004). In the unusual case that one individual makes the same solution more than once in one day, simply insert numbers after the first three letters to indicate the order in which they were made. For example, the second batch of TAE buffer that Evelyn Goss made on November 6, 2004 would have the control number: TAE-2-EG-041106. 109 6. Fill out all sections of the solution prep form (see Appendix A for an example). If a field contains information that is not applicable, enter “N/A” in that field. Enter all information (except the amount of each chemical used) before preparing the solution. Enter the amounts used after weighing and delivering the chemical(s) to the preparation vessel. 7. After preparing the solution and transferring solution and label to the storage vessel (if necessary), complete the label by including the following information: Solution prep control number Any special instructions, such as expiration date or storage conditions Safety warnings if the solution contains hazardous materials Course number and semester (if applicable) 8. File the solution prep form alphabetically in the top drawer of the black file cabinet. If you are a student taking a course, hand into your instructor, who will make copies as needed and give the original back to you for your lab report. The instructor should always keep a copy on file in the lab. Never enter the amount of a chemical used until you have actually measured and added the quantity. Do not enter the calculated mass or volume; only enter the actual, measured quantity. 110 Appendix A SOLUTION PREP FORM Name of Solution/Media: Amount prepared: Control # TAE-EG-041106 50X TAE Buffer 1L Preparer(s): Evelyn Goss Component Brand/lot # (Vendor) Trizma base Preparation Date: November 6, 2004 FW or initial concentration Amount used Final concentration Sigma/022385 Storage conditions/ date received RT/031505 121.1 g/mol 242 g 2M Glacial acetic acid EMD/43DBB31 RT/053099 60.05 g/mol 57.1 mL 5.71% EDTA Fisher/ 960654A RT/012502 372.24 g/mol 37.2 g 100 mM Balance used #5 Calibration status OK pH meter used N/A Calibration status N/A Initial pH 8.5 Final pH 8.5 Adjusted pH with N/A Prep temperature RT Sterilization procedure/ sterility testing N/A Storage conditions RT Calculations/Comments: See protocol on page A1-31 of Short Protocols in Molecular Biology, Vol.2, 5 th Ed., Ausubel, et al. 111 SOP SPC-001 GENESYS 10 SPECTROPHOTOMETER Revision A, June 15, 2011 Title: Use and Maintenance of the Genesys 10 Spectrophotometer 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the Genesys 10 Spectrophotometer. 2.0 SUMMARY OF METHOD PART A: BASIC ABSORBANCE, TRANSMITTANCE, AND CONCENTRATION READINGS 1. Turn on the spectrophotometer using the power switch on the back of the instrument. 2. Wait for the instrument to initialize and complete internal tests. 3. From the home screen, press the softkey at the bottom right labeled “Basic ATC.” 4. Press the softkey labeled “Set nm.” Enter the desired wavelength using the numeric keypad and press Enter. 5. Choose cuvettes appropriate for the wavelength. Make sure they are clean on the inside and outside and free from scratches or stains. 6. Wearing gloves, fill one clean cuvette at least halfway with the solution to be used as a blank. 7. Fill another cuvette at least halfway with the solution to be tested for absorbance. If there is a shortage of cuvettes, the blank can be measured first, then removed, and the blank solution replaced with the solution to be tested. 8. Carefully wipe the optically clear sides of the cuvettes with a Kimwipe or lens paper to remove any drops of liquid, dust, or fingerprints. This option will allow the user to make basic absorbance measurements at a given wavelength. Some plastics are not UV-transparent and would not be useful for UV absorbance readings. The blank solution should be identical to or as similar as possible to the solution in which the compound to be measured is suspended. This is usually water or an aqueous buffer. Orient the cuvette such that the optically clear sides are aligned with the light path. It is best to handle cuvettes by the optically opaque sides to avoid leaving any residue on the clear sides. 112 9. Open the sample compartment. Insert the blank cuvette in the sample holder labeled “B”. Orient the cuvette such that the optically clear sides are aligned with the light path. 10. Insert the sample cuvette in the first sample holder, labeled “1”. If there are additional samples and enough cuvettes to hold them, insert these in the other four sample holders and note their positions. 11. Close the sample compartment. 12. Locate the numbered sample selection keys (arranged in a circle on the keypad). Press “B” to select the blank. 13. Press the softkey labeled “Measure blank”. The display should read 0.000A. 14. Press the sample selection key with the number of the sample you wish to analyze. The sample holder carousel will rotate and the instrument will analyze the selected sample, displaying the absorbance. 15. If the absorbance is greater than 2.0, the display will show an error message stating that the sample is outside the range of the instrument. This will require you to dilute the sample to gain an accurate reading. 16. Repeat steps 14-15 with all other samples. 17. When you are finished, clean the cuvettes by first emptying them, then flushing with several changes of purified water. Next, flush with several changes of 70-95% ethanol. Finally, dry upside down on a paper towel before storing in a safe place. PART B: DNA ANALYSIS BY SCANNING 1. From the main menu, highlight “Nucleic Acid Tests” and press Enter. 2. Select “DNA with Scan 260/230”. 3. Parameters will display; press the softkey labeled “Run Test”. A 1/10 dilution is a good place to start. Use the blank solution to dilute the sample. If the absorbance is still too high, dilute again and repeat until an accurate measurement is obtained. Then, multiply the absorbance reading by the dilution factor to obtain the absorbance of the original concentration. Never use any abrasive cleansers, soaps, or brushes on cuvettes. This can scratch them or leave a contaminating residue. Disposable plastic cuvettes can be used a few times before they become scratched or stained and need to be discarded. Clean these carefully and they will last for several uses. Quartz cuvettes look like glass and are made to have a long life. Treat these with special care because they are very expensive. They will last many years if used carefully. 113 4. Insert a cuvette filled with the blank solution appropriate to your experiment into the sample holder labeled “B”. 5. Press the softkey labeled “Collect Baseline” and the instrument will collect baseline data. 6. When baseline collection is complete, remove the blank cuvette. 7. Insert a cuvette filled with your sample into one of the numbered sample holders. 8. Press the softkey labeled “Collect Data” and the instrument will scan the sample. 9. When complete, the instrument will print a record of the scan. Collect the printout and press Escape to return to the main menu. 10. Remove all samples from the sample compartment and clean the cuvettes thoroughly (see 17, Part A). 11. If you are the last to use the spectrophotometer, power off the instrument. PART C: KINETICS 1. From the main menu, select “Kinetics” and press Enter. 2. Select “Stored Tests” and press Enter to recall a stored test. Or, change the parameters on the Kinetics home screen to create a new test, then press the “Save Test” softkey to save it. 3. With the selected test open, press the “Run Test” softkey to start the test. 4. Insert a cuvette filled with the blank solution appropriate to your experiment into the sample holder labeled “B”. 5. Press the softkey labeled “Collect Baseline” and the instrument will collect baseline data. 114 6. When baseline collection is complete, remove the blank cuvette. 7. Insert a cuvette filled with your sample into one of the numbered sample holders. 8. Press the softkey labeled “Collect Data” and the instrument will begin the test. 9. If you did not select Auto Print while defining parameters for the test, you will need to press the Print button to obtain a printout of your results. 115 SOP SPC-002 BioMate 3 SPECTROPHOTOMETER Revision A, June 15, 2011 Title: Use and Maintenance of the BioMate 3 Spectrophotometer 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use and maintenance of the BioMate 3 Spectrophotometer. 2.0 SUMMARY OF METHOD MATERIALS BioMate 3 Spectrophotometer Cuvettes PART A: BASIC ABSORBANCE, TRANSMITTANCE, AND CONCENTRATION READINGS 1. Turn on the spectrophotometer using the power switch on the back of the instrument. 2. Wait for the instrument to initialize and complete internal tests. 3. From the home screen, press the softkey at the bottom right labeled “Basic ATC.” 4. Press the softkey labeled “Set nm.” Enter the desired wavelength using the numeric keypad and press Enter. 5. Choose cuvettes appropriate for the wavelength. Make sure they are clean on the inside and outside and free from scratches or stains. 6. Wearing gloves, fill one clean cuvette at least halfway with the solution to be used as a blank. 7. Fill another cuvette at least halfway with the solution to be tested for absorbance. If there is a shortage of cuvettes, the blank can be measured first, then removed, and the blank solution replaced with the solution to be tested. 8. Carefully wipe the optically clear sides of the cuvettes with a Kimwipe or lens paper This option will allow the user to make basic absorbance measurements at a given wavelength. Some plastics are not UV-transparent and would not be useful for UV absorbance readings. The blank solution should be identical to or as similar as possible to the solution in which the compound to be measured is suspended. This is usually water or an aqueous buffer. It is best to handle cuvettes by the optically 116 to remove any drops of liquid, dust, or fingerprints. opaque sides to avoid leaving any residue on the clear sides. 9. Open the sample compartment. Insert the blank cuvette in the sample holder. Orient the cuvette such that the optically clear sides are aligned with the light path. 10. Close the sample compartment. 11. Press the softkey labeled “Measure blank”. The display should read 0.000A. 12. Remove the blank cuvette and insert a cuvette filled with your sample. The instrument will display the measured absorbance. 13. If the absorbance is greater than 2.0, the display will show an error message stating that the sample is outside the range of the instrument. This will require you to dilute the sample to gain an accurate reading. 14. Repeat steps 14-15 with all other samples. 15. When you are finished, clean the cuvettes by first emptying them, then flushing with several changes of purified water. Next, flush with several changes of 70-95% ethanol. Finally, dry upside down on a paper towel before storing in a safe place. 16. If you are the last to use the spectrophotometer, power off the instrument. PART B: DNA ANALYSIS BY SCANNING 1. From the main menu, highlight “Nucleic Acid Tests” and press Enter. 2. Select “DNA with Scan 260/230”. 3. Parameters will display; press the softkey labeled “Run Test”. 4. Insert a cuvette filled with the blank solution appropriate to your experiment into the sample holder. A 1/10 dilution is a good place to start. Use the blank solution to dilute the sample. If the absorbance is still too high, dilute again and repeat until an accurate measurement is obtained. Then, multiply the absorbance reading by the dilution factor to obtain the absorbance of the original concentration. Never use any abrasive cleansers, soaps, or brushes on cuvettes. This can scratch them or leave a contaminating residue. Disposable plastic cuvettes can be used a few times before they become scratched or stained and need to be discarded. Clean these carefully and they will last for several uses. Quartz cuvettes look like glass and are made to have a long life. Treat these with special care because they are very expensive. They will last many years if used carefully. 117 5. Press the softkey labeled “Collect Baseline” and allow the instrument to collect baseline data. 6. When baseline collection is complete, remove the blank cuvette. 7. Insert a cuvette filled with your sample into the sample holder. 8. Press the softkey labeled “Collect Data” and allow the instrument to scan the sample. 9. When complete, the instrument will print a record of the scan. Collect the printout and press Escape to return to the main menu. 10. Remove the sample from the sample compartment and clean all cuvettes thoroughly (see 17, Part A). 11. If you are the last to use the spectrophotometer, power off the instrument. PART C: KINETICS 1. From the main menu, select “Kinetics” and press Enter. 2. Select “Stored Tests” and press Enter to recall a stored test. Or, change the parameters on the Kinetics home screen to create a new test, then press the “Save Test” softkey to save it. 3. With the selected test open, press the “Run Test” softkey to start the test. 4. Insert a cuvette filled with the blank solution appropriate to your experiment into the sample holder labeled “B”. 5. Press the softkey labeled “Collect Baseline” and the instrument will collect baseline data. 6. When baseline collection is complete, remove the blank cuvette. 7. Insert a cuvette filled with your sample into 118 one of the numbered sample holders. 8. Press the softkey labeled “Collect Data” and the instrument will begin the test. 9. If you did not select Auto Print while defining parameters for the test, you will need to press the Print button to obtain a printout of your results. 119 SOP SPC-003 NANODROP 1000 SPECTROPHOTOMETER Title: Operation of NanoDrop 1000 Spectrophotometer 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the NanoDrop 1000 Spectrophotometer. 2.0 SUMMARY OF METHOD MATERIALS NanoDrop 1000 spectrophotometer Attached computer with ND1000 software Kimwipes, DI water, p2 pipette PART A: OPERATION IMPORTANT: do not handle the fiber optic cable on the NanoDrop. This can damage it and cause problems with the instrument. When lifting and lowering the pedestal, handle only by the metal part at the bottom. Keep the instrument away from objects that could fall on or snag the cable to prevent damage. 1. Open the ND1000 software on the attached computer and select the analysis module of interest (nucleic acid, protein, etc). 2. Follow prompts to initialize the spectrophotometer by pipetting 2 uL of DI water onto the bottom pedestal and clicking OK. When this is finished, wipe the water off with a kimwipe. 3. Establish a reference or blank using the appropriate buffer. Pipette 1-2 µL of blanking buffer onto the bottom pedestal and click the Blank button. Wipe the blank off with a kimwipe. 4. Pipette 1-2 µL of the sample onto the bottom pedestal and click the Measure button. Wipe the sample off with a kimwipe. 5. Measure other samples by repeating steps 3 and 4. 6. If desired, pipette 1-2 µL of deionized water onto the bottom pedestal and wipe with a Kimwipe to clean the sample holder. DO NOT use detergents or alcohol to clean the pedestals, as their use may result in pedestals becoming unconditioned. 120 SOP SPC-004 LAMBDA BIO+ SPECTROPHOTOMETER Revision A, June 8, 2011 Title: Use and Maintenance of the Lambda Bio+ Spectrophotometer 1.0 SCOPE AND APPLICATION: This SOP outlines the procedure for use and maintenance of the Lambda Bio+ Spectrophotometer. 2.0 SUMMARY OF METHOD MATERIALS Lambda Bio+ Spectrophotometer Cuvettes with 15 mm sample window NOTE: The beam height of this instrument is 15 mm. You must either fill the cuvette at least 15 mm high, or use a 15 mm height adaptor. When using a UV wavelength, you must use UV-transparent cuvettes. PART A: SINGLE WAVELENGTH READINGS 1. Turn on the spectrophotometer by pressing the power button. 2. Wait for the instrument to initialize and complete internal tests. The default home page will be displayed. Some plastics are not UV-transparent and would not be useful for UV absorbance readings. Quartz cuvettes are UVtransparent. Make sure that the sides of the cuvette facing the sides of the instrument are optically transparent. The light path travels from right to left across the instrument. It is best to handle cuvettes by the optically opaque sides to avoid leaving any residue on the clear sides. 3. Press 1 on the number keypad to choose Single Wavelength. 4. Enter the wavelength you would like to use, then press the green ► button on the keypad to accept. 5. Insert a cuvette containing the appropriate blank solution. Press the 0A/100%T button on the keypad to take a blank reading. 6. Remove the blank cuvette and insert a cuvette containing the sample. Press the green ► button to take a reading. The absorbance will be displayed. 7. If the absorbance value of a sample is above the range of the instrument, you will need to dilute the sample. 8. Repeat with any other samples. The blank solution should be identical to or as similar as possible to the solution in which the compound to be measured is suspended. This is usually water or an aqueous buffer. A 1/10 dilution is a good place to start. Use the blank solution to dilute the 121 9. When you are finished, clean the cuvettes by first emptying them, then flushing with several changes of purified water. Next, flush with several changes of 70-95% ethanol. Finally, dry upside down on a paper towel before storing in a safe place. 10. If you are the last to use the spectrophotometer, power off the instrument. PART B: MULTI-WAVELENGTH READINGS 1. Follow the procedure for single wavelength readings with these two modifications: 2. In Step 3, choose 2 for multi-wavelength readings. 3. In Step 4, enter two or more wavelengths. sample. If the absorbance is still too high, dilute again and repeat until an accurate measurement is obtained. Then, multiply the absorbance reading by the dilution factor to obtain the absorbance of the original concentration. Never use any abrasive cleansers, soaps, or brushes on cuvettes. This can scratch them or leave a contaminating residue. Disposable plastic cuvettes can be used a few times before they become scratched or stained and need to be discarded. Clean these carefully and they will last for several uses. Quartz cuvettes look like glass and are made to have a long life. Treat these with special care because they are very expensive. They will last many years if used carefully. PART C: ABSORBANCE SPECTRUM 1. Choose 3 for Spectrum in the default home page. 2. Enter the start and end wavelengths and press the green ► button. 3. Insert a cuvette containing the appropriate blank solution. Press the 0A/100%T button on the keypad to take a blank reading. 4. Remove the blank cuvette and insert a cuvette containing the sample. Press the green ► button to take a reading. The absorbance spectrum will be displayed. 5. Repeat with any other samples. 6. When you are finished, clean the cuvettes by first emptying them, then flushing with several changes of purified water. Next, flush with several changes of 70-95% ethanol. Finally, dry upside down on a paper towel before storing in a safe place. The blank solution should be identical to or as similar as possible to the solution in which the compound to be measured is suspended. This is usually water or an aqueous buffer. 7. If you are the last to use the spectrophotometer, power off the instrument. PART C: KINETICS A 1/10 dilution is a good place to start. 122 1. From the Standard Methods home screen, press 6 for Kinetics. 2. Enter the wavelength, delay time (if any), duration time, and interval length using the numeric keypad and arrows. 3. Press the green ► button to continue. 4. Select the measurement mode using the left and right arrows (you will most likely want to select “Slope”). Press the down arrow to get to units, then enter the units manually or press the Options button to choose from a list of units. Press the down arrow and change the “Factor” setting if you need to multiply results by a dilution factor. 5. Press the green ► button to continue. 6. Insert a cuvette containing the reference sample and press the 0A/100%T button to take a blank reading. 7. Insert a cuvette containing the sample and press the green ► button to begin measuring. The window will display a real-time plot of absorbance. 8. Press the Options button to print, save, or change view settings. Use the blank solution to dilute the sample. If the absorbance is still too high, dilute again and repeat until an accurate measurement is obtained. Then, multiply the absorbance reading by the dilution factor to obtain the absorbance of the original concentration. Never use any abrasive cleansers, soaps, or brushes on cuvettes. This can scratch them or leave a contaminating residue. Disposable plastic cuvettes can be used a few times before they become scratched or stained and need to be discarded. Clean these carefully and they will last for several uses. Quartz cuvettes look like glass and are made to have a long life. Treat these with special care because they are very expensive. They will last many years if used carefully. 123 SOP SPC-008 NANODROP 2000 SPECTROPHOTOMETER Revision A, June 8, 2011 Title: Operation of the NanoDrop 2000 Spectrophotometer 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation of the NanoDrop 2000 Spectrophotometer. 2.0 SUMMARY OF METHOD MATERIALS Nanodrop 2000 Spectrophotometer Attached computer with ND2000 software P2 or P10 pipettor and tips Deionized water and Kimwipes PART A: Using the NanoDrop 2000 1. Open the ND2000 software on the desktop of the attached computer. If asked for a password, click Enter without typing a password. 2. The main menu will open and you will be able to choose from a number of methods, including Nucleic Acid, Protein, etc. Select the appropriate method and it will open. 3. You will be asked if you would like to open the previously used workbook; choose No. 4. Make sure arm is down and click ok to start the Wavelength Verification. 5. Go to File and New Workbook. Name and save your workbook; all samples will now be saved to your workbook until you select a new workbook. This provides a simple way to tabulate and report multiple measurements. 6. Establish a reference or blank using the appropriate buffer or water (whatever your sample is dissolved in). Pipette 1-2uL of blank solution onto the bottom pedestal and click “BLANK”, located at Note: the blank reading will not display any values or a spectrum. These will not appear until you perform a measurement. If you blank again after taking a measurement, the data and spectrum from the previous measurement will remain on the screen. To verify the blank, click Measure to record your blank solution as a sample. A spectrum 124 the top left corner of the screen. and absorbance and concentration values will appear on the display. 7. Wipe upper and lower pedestals with a Kimwipe. 8. Pipette 1-2uL of the sample onto the bottom pedestal and click “MEASURE” to obtain an absorbance. You may name the sample in the Sample ID field. 9. Wipe upper and lower pedestals with a Kimwipe. 10. Measure other samples by repeating steps 8 and 9. 11. If desired, pipette 1-2uL of deionized water onto the bottom pedestal and wipe with a Kimwipe to clean the sample holder. DO NOT use detergents or alcohol to clean the pedestals, as this may result in pedestals becoming unconditioned. 125 SOP TAC-001 TACHOMETER Revision A, June 8, 2011 Title: Use and Maintenance of Tachometer 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for use of a tachometer to measure the speed of a centrifuge. 2.0 SUMMARY OF METHOD MATERIALS Tachometer Centrifuge Reflective tape, Sharpie PART A: SETUP 1. Locate the part of the centrifuge that will show through the transparent viewing window on the centrifuge lid. Usually, this is the very center of the rotor. The tachometer works by shining a light onto a visible, rotating part of the centrifuge rotor. When there is a reflective object on this part, the light will be reflected back in bursts at the same frequency as the rotor is moving (in rpm). 2. Open the centrifuge and observe this part. If it is reflective, you will need to color it black with a marker. 3. Attach a strip or triangle of reflective tape to one side of the visible part. PART B: OPERATION 1. Close the centrifuge and start a run. 2. Set the tachometer to Photo settting by moving the toggle switch all the way to the right. 3. Hold the tachometer with the light beam shining down into the viewing window. There is a foot on the tachometer that will allow you to rest it on the surface of the centrifuge. 4. Hold the Measure button (on the side of the tachometer). Numbers will appear on the LCD screen. NOTE: the tachometer should be stored without batteries because they become run down if stored in the instrument. Please insert batteries before using. 126 5. The reading should be approximately the same as the speed setting on the centrifuge. 6. When finished using the tachometer, remove the batteries because they will be run down if stored in the instrument. PART C: MAINTANANCE 1. Once per year, the tachometer should be sent to the Control Company for calibration. See the calibration document in the box for the address. If the reading is twice the speed settting on the centrifuge, you have most likely situated the reflective tape so that two burst of light are reflected per rotation. Fix the tape so that only one burst of light will be reflected per rotation. 127 SOP VAC-001 INTEGRA VACUSAFE ASPIRATOR Revision A, August 16, 2011 Title: Integra VacuSafe Aspirator 1.0 SCOPE AND APPLICATION: This SOP outlines the setup, use, and cleaning of the Integra VacuSafe Aspiration System for cell culture. 2.0 SUMMARY OF METHOD Materials VacuSafe aspiration system Sterile pipettes Operation 1. Add approximately 100 mL of 10% bleach to the VacuSafe bottle and replace the lid. 2. Turn on the VacuSafe pump. 3. Grasp the VacuSafe controller and insert a sterile pipette. 4. Place the tip of the pipette in the liquid to be aspirated and depress and hold the button on the side of the controller to open the vacuum. 5. Liquid should flow into the tubing and into the bottle. To stop aspirating, release the button. 6. When finished, remove the pipette from the controller. Flush the controller and tubing with air by depressing the button and allowing air to flow through the system. Turn off the vacuum pump. 7. To empty the bottle, remove the small, clear silicone cap from the third hold on the lid of the bottle. This will allow air into the system and you will be able to unscrew the lid. COMMENTS Bleach is used in the bottle to kill any cells so the flow-through will be decontaminated before disposal. Make sure the VacuSafe is somewhere where it will not be knocked over, but is convenient for use. If the pipette is difficult to insert, try wetting the end with alcohol. 128 SOP WPS-001 OPERATION AND CARE OF RoDi WATER PURIFICATION SYSTEM Revision A, June 8, 2011 1.0 SCOPE AND APPLICATION: This SOP outlines the policy and procedure for operation and care of the RoDi water purification system. 2.0 SUMMARY OF METHOD MODES OF OPERATION 1. If RoDi unit is in Run mode, you will see a number (such as “17.6”) on the digital display. This is the resistivity of the water in megaohms•cm and indicates the purity of the water. While in Run mode, water is being purified continuously. Unit should be in Run mode to dispense water. 2. If RoDi unit is in Standby mode, you will see “Sby” on the LED readout. The unit should be left in Standby mode when not in use to conserve the UV light bulb, RO membrane and cartridges. COMMENTS The more pure the water, the higher its resistivity will be. In Standby mode, the pump will operate for 10 minutes out of every hour, and the UV light will operate for 10 minutes out of every four hours. 3. If RoDi unit is in Idle mode, you will see “IdL” on the LED readout. Idle mode halts purification until Start/Stop is pressed and unit enters Run mode. Idle mode should be avoided to ensure a supply of high purity water at all times. PROCEDURE: OPERATION 1. Locate the container into which you intend to dispense purified water. 2. Identify the operational mode of the unit by using the guidelines in the above section. If it is not in Run mode, press Start/Stop. A number indicating the resistivity of the water should appear. 3. If necessary, allow the resistivity to rise until water has reached the desired purity. A resistivity of at least 16 megaohms•cm is adequate for most Glass is the best storage material for water containers, especially for long-term storage, because it does not leach materials like plastic does. Unit should be left on at all times. If unit is turned off, locate the power switch on the back of the unit and turn on. Notify the lab assistant if unit is turned off. 129 applications. 4. Position the container under the drawoff valve without touching the valve to avoid contamination of the unit. 5. Press down on the draw-off valve lever to dispense water. Lever will click down and stay in position. When finished, move the lever up to the off position. 6. Press Standby to put the unit in Standby mode. This will slow the purification processes to conserve the cartridges, membrane and UV bulb, while still ensuring a supply of high purity water for when Run mode is activated again. TROUBLESHOOTING If an error message appears on the display or if you encounter any problems with the unit, see the troubleshooting guide starting on page 41 of the manual. MAINTENANCE 1. Every six months, the reverse osmosis prefilter should be replaced. The Replace RO Prefilter LED (small red light) will turn on every six months. 2. If residual deposits are evident in feedwater reservoir, or if a new 0.2 micron final filter clogs rapidly, the reservoir should be cleaned according to directions on page 28 of the manual, and cartridges should be replaced. 3. When recirculated water will not rinse up to desired purity level, replace cartridges. If problem persists, clean the resistivity cell according to directions on page 30 of the manual. 4. If low RO purity LED is illuminated even after an hour of rinse-up, replace the RO membrane. 130 Record of Revisions to Standard Operating Procedures Table 1: Updates in the 9th Edition, Fall 2012 SOP Title Changes Justification CAM-001 (VistaVision Microscope Camera ELC-001 (Bio-Rad Mini-Sub GT cells) FCT-001 (Accuri Flow Cytometer) FLU-001 (Quantech Fluorometer) FLU-003 (NanoDrop 3300 Fluorospectrophotometer) PCR-002 (Bio-Rad iCycler for endpoint PCR) PCR-003 (Stratagene Mx4000) PCR-006 (Bio-Rad iCycler for Real-time qPCR) SPC-005 (GE Nanovue Spectrophotometer) SPC-007 (Bio-Rad SmartSpec) ELC-004 (Edvotek Electrophoresis Chambers AUT-001 (VWR/Thermo Scientific Sterilemax Autoclave) SOP removed. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment rarely used in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment no longer in use in our labs. Equipment is frequently used in our labs. The same model Sterilemax autoclave was sold by VWR and Thermo Scientific and we own one of each. Renamed to remove ambiguity. A support technician from Thermo suggested we use deionized water instead of tap. Incorrect label was left from using MT balance SOP as a template. BAL-005 (Ohaus Scout Pro Balance) SOP removed. SOP removed. SOP removed. SOP removed. SOP removed. SOP removed. SOP removed. SOP removed. SOP removed. SOP added. Title of SOP and name of autoclave changed from “VWR” to “VWR/Thermo Scientific Sterilemax). Changed “tap water” to “deionized water.” Corrected name of balance in the title from “Mettler-Toledo PL1502-S” to “Ohaus Scout Pro SP402.” Name of Editor Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss Evelyn Goss



