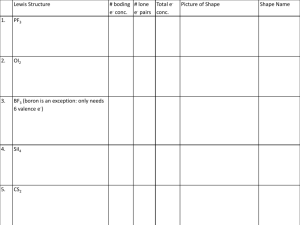
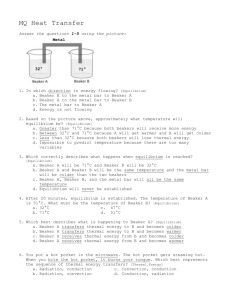
More Chemistry Writing Prompts
advertisement

WRITING PROMPTS - CHEMISTRY Respond to the following statement: "All substances are either soluble or insoluble. If I put some stuff in water and nothing appears to happen, then it must be completely insoluble. A solubility chart can tell me this information and it is always correct." Explain how to calculate the pH of a base solution. The molecules BF3 and NH3 both have covalent bonds. Explain why BF3 is described as a trigonal planer shape while NH3 is described as a trigonal pyramidal shape. Write a paragraph explaining the differences between a real gas and an ideal gas. Use the ideas from the kinetic molecular theory, and give examples of when an ideal gas may be described as a real gas. After reading Passage 1, “The Science of Chemistry,” do you generally think of chemicals in negative terms as described in paragraph three or do you think of them with a more positive point of view as described in paragraph four? Why? Please explain your point of view with a paragraph. Use the “Writing Across the Curriculum: template to help organize your thoughts before beginning your paragraph. Explain how the structures of the four types of organic molecules relate to function. Explain the difference between quantitative and qualitative data. In your exploration, give examples of the two types of data. A student mixed two substances together and concluded that a chemical reaction had taken place. Explain how the student was able to conclude that a chemical reaction occurred. In your explanation, be sure to include • evidence that would support the student’s conclusion Compare the properties of oil to the properties of the elements in oil. In your comparison, be sure to include • the properties of oil • the properties of the elements in oil • the motion of the molecules in oil, carbon, and hydrogen • Explain how heat energy affects a substance. In your explanation, be sure to include • the temperatures at which a substance changes states of matter After the beaker containing Mixture 4 was sealed, the iron filings changed color. Compare the mass of the mixture in the sealed beaker to the masses of the iron filings and water before they were mixed. In your comparison, be sure to include • the law of conservation of matter • evidence from the data table