Molar Concentrations and Solutions
advertisement

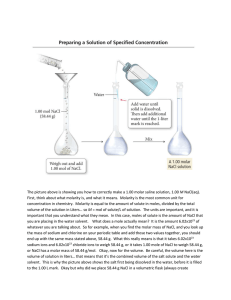
Molar Concentrations and Solutions The concentration of a substance in solution is the amount of the substance in a given volume of the solution. The term concentrated is used to describe a solution with a relatively high concentration of solutes (dissolved substances). To describe a relatively low amount of solute in a solvent, we use the term dilute. These terms are not clearly defined so they have no precise meanings. The term saturated solution is more precise. It means that the solvent cannot dissolve anymore solute so that adding any more solute will result in a precipitate (visible, undissolved solid). The molar concentration, or molarity, is defined as the number of moles in 1 L of solution measured in mol/L or M (read molar). We can use the following formula to express molar concentration. Molar concentration = moles/volume c = n/V c = molar concentration (mol/L or M) n = number of mole (mol) V = volume (L) Example: If a 1.0 L of solution contains 2.5 mol of NaCl, the molar concentration can be expressed in the following ways. Molar concentration of NaCl = [NaCl] = 2.5 mol/L = 2.5 M The molarity of NaCl is 2.5 molar Practice: What is the [NaCl] in a solution containing 5.12 g of NaCl in 250.0 mL of solution? Practice: What mass of NaOH is contained in 3.50 L of 0.200 M NaOH? Practice: What is the actual experimental procedure you would use to prepare 2.75 L of 0.0120 M NaOH? Dilutions Dilution is the act of making a solution less concentrated. Since concentration is the number of moles per volume, the more volume of solvent we have, the less concentrated, or more dilute, the solution becomes. By adding more volume of solvent, the number of moles of the solute does not change. c = n/V So n does not change but V increases, resulting in a decreased c. Example: If 200.0 mL of 0.500 M NaCl is added to 300.0 mL of water, what is the resulting [NaCl] in the mixture? Practice: If 20.0 mL of 0.75 M HBr is diluted to a total volume of 90.0 mL, what is [HBr] in the resulting solution? Mixing Two Solutions of the Same Chemical with Different Concentrations When performing calculations with concentrations, a good strategy is to always calculate the number of moles of the substances in question and then divide by the total volume for the final concentration. Example: If 300.0 mL of 0.250 NaCl is added to 500.0 mL of 0.100 M NaCl, what is the resulting [NaCl] in the mixture? First find the number of moles of NaCl in each solution then divide by the total volume (in L) to get the final concentration. You can check to see that the answer makes sense because the concentration should be between that of the two original solutions. Practice: What is [KOH] resulting from mixing 55 mL of 0.15 M KOH and 75 mL of 0.25 M KOH?