pbi12095-sup-0001-FiguresS1-S7
advertisement

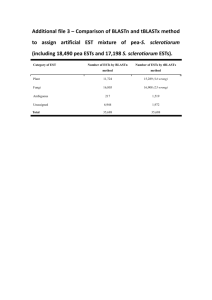
Figure S1. Structure of glucosinolates. R indicates various side chains. Glucoerucin (4MTB) and glucoraphanin (4MSB) are shown as representatives of methylthio- and methylsulfinyl-GSLs, respectively. Gluc, glucose moiety. Figure S2. Glucosinolate content in kale leaves. The GSL profile in kale (cv. Haikuroppu) leaves was analyzed by HPLC-QMS (see Appendix S1). Data of detected GSLs from 4 biological replicates (1–4) are shown. 2OHB, 2-hydroxy-3-butenyl GSL. Figure S3. Characteristics of kale FL-cDNA libraries. Distribution of the length of 5′-end ESTs (a) and 3′-end ESTs (b). (c) Redundancy rate of kale FL-cDNA libraries. Evaluation of the cover ratio of kale FL-cDNA libraries to whole genes in kale was estimated by annotation of the kale FL-cDNA by AGI codes at 3 points. (d) Proportion of FL-cDNA clones from different origins. FL-cDNA clones were distinguished by their tissue origins by searching for signature sequences in corresponding 3′-end ESTs. Any EST whose origin could not be determined was removed. (e) Frequency of kale FL-cDNA clones assigned to identical AGI codes. FL-cDNA clones assigned with the same AGI code were assembled to make clusters. Figure S4. Result of BLAST search with kale 3′-end ESTs. The kale 3′-end ESTs were queried using Blast2go under default settings. Figure S5. Glucosinolate profiles in T3 leaves of BoMYB29-overexpressing lines. Five T3 seedlings from representative T2 lines were grown on 1/2 MS plates without antibiotics for 3 weeks. The rosette leaves of each individual were divided into 2 groups, and one was subjected to GSL analysis using UPLC-TQMS. The vertical axis indicates relative peak area of the GSL, which was normalized by an internal standard peak area and divided by sample fresh weight (mg). Numbers from i1 to i20 indicate each seedling and samples underlined in red were those used for gene expression analysis shown in Figure 5. Accumulation of (a) all GSL species, (b) short-chain aliphatic GSLs, (c) long-chain aliphatic GSLs, and (d) indole GSLs. T3 individuals i3 and i9 from Lines #2–5 and #5–2, respectively, were considered to be homozygous. i6 from Line #5–2 appeared to contain a null allele. Figure S6. GSL contents in T1 leaves of myb28myb29 dKO mutants transformed with BoMYB29. Fifty-four T1 transformants selected on a hygromycin-containing plate were transferred to soil and cultivated for 3 weeks. Detached leaves from the 3-week-old transformants were subjected to GSL analysis using UPLC-TQMS. T1 plants transformed with the CaMV 35S RNA promoter::GUS gene were used as the vector control. In this experiment, the wild-type plant was not used as control because young seedlings needed to be grown on hygromycin-containing medium for selection. The numbers on the horizontal axis indicate individual T1 transgenic plants. Figure S7. Schematic representation of gene-specific primers for AtMYB29. Black and red arrows indicate primer sequence positions. The 3′ UTR primer set (black arrows) could only detect endogenous AtMYB29 since the transcript from the transgene sequence does not contain a 3′ UTR. The AtMYB29-exon primer set (red arrows) could amplify transcripts from both endogenous and introduced AtMYB29.