Sedatives and Hypnot..
advertisement

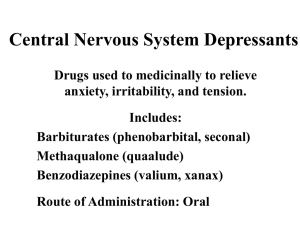
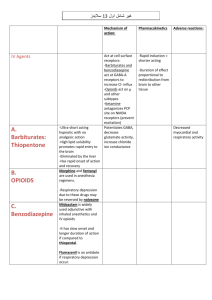
Sedatives and Hypnotics Sedatives and hypnotics are CNS depressants associated with the reduction of anxiety (anxiolytic) and induction of sleep. A large number of compounds related to different chemical groups are currently used as sedative and hypnotics. CNS depression ranges from mild sedation to sleep, depending on route of administration and dose (small doses sedation, ↑ doses hypnosis and ↑↑ doses surgical anaesthesia). Sedation: It is mild depression without causing drowsiness, used in emotional strain, excitation, hypertension, convulsions, preanesthesia and potentiation of analgesic drugs. Hypnosis: It is strong depression causing sleep, used in insomnia. The drugs used as hypnotics are often used as sedatives and anxiolytics (by low dose) but not all anxiolytics cause sedation and hypnosis. Ideal Hypnotic Properties: 1- It should decrease the consciousness for sleeping without lingering effects (sleep induction and maintenance). 2- It should have no potential for or stopping (arresting) respiration. 3- It should produce no abuse, addiction, tolerance or dependence (as in barbiturate tolerance). 1 Sleep Factors: These are factors which control sleep, and any imbalance in them make disturbances and difficulty to sleep. 1- Catecholamines ( sleep). 2- Serotonin (↑ sleep). 3- Histamine ( sleep). 4- Acetylcholine (↑ sleep). 5- Growth hormone (↑ sleep). 6- Melatonin (↑ sleep). Classification of Hypnotics: A. Barbiturates (barbituric acid derivatives). B. Non-barbiturates. A- Barbituric Acid Derivatives Barbiturates were among the most frequently used sedative and hypnotic drugs. Also, they are used as anticonvulsants. These days their use are minimized (as sedative/hypnotic) due to their tolerance, dependence and toxicity (respiratory depression) and interaction with other drugs. Mechanism of Action: Barbiturates enhance GABA binding ↑ chloride influx inhibitory action (sedation, hypnosis and anticonvulsant actions). pKa and Structure of Barbiturates: In 1951, Sandberg postulated that to possess a good hypnotic activity, a barbiturate must be a weak acid and must have a lipid/H2O partition 2 coefficient between certain limits to provide good lipid solubility. The CNS depressant barbiturates are mainly the 5,5-disubstituted barbiturates, and 1,5,5-trisubstituted barbiturates. Ideal Hypnotic Activity: 1- The barbiturate should be weak acid. 2- ↑ Partition coefficient because ↑ pKa acidity polarity↑ lipophilicity ↑ crossing BBB. The acidity of barbiturates in aqueous solution depends on the number of substituents attached to barbituric acid at 1, 3 and 5, positions. General Method of Preparation of Barbiturates: The common method of synthesis of 5,5-disubstituted barbiturates involves the condensation of disubstituted diethyl malonate and urea or thiourea in absolute ethanol, in the presence of sodium ethoxide. The substituted diethyl malonate required for this reaction is usually prepared by treatment of diethyl malonate with calculated amount of sodium ethoxide in absolute ethanol to give the monosodium derivative which is readily converted to the monoalkyl derivative via interaction with the appropriate alkyl halide. This process is repeated to introduce the second alkyl group. 3 Structure-Activity Relationships (SAR): 1- Both hydrogens at position 5 of barbituric acid must be substituted for maximal activity. This is likely due to susceptibility to rapid metabolic attack at this position. 2- Increasing the length of an alkyl group at position 5 increases the potency (up to 5-6 carbons), beyond that CNS depressant action decreases and convulsion action may result. 3- Branching and/or unsaturation in the alkyl group at position 5 ↑ CNS depressant activity with shorter duration (↑ lipid solubility). 4- A phenyl group at position 5 ⇒ anticonvulsant activity as it ↑ CNS depressant activity and prolong the duration of action. 4 5- Halogenation to the alkyl substituents at position 5 ↑ potency. 6- Substitution on one imide by alkyl group ⇒↑ lipid solubility ⇒ rapid onset and short duration of action ⇒↑ anticonvulsant / ↑ anesthetic activities. 7- Substitution to both N1 and N3 by alkyl groups ⇒ No acidity ⇒ inactive agents. 8- Introduction of a polar functional group, e.g., OH, C=O, amino in the alkyl group at position 5 destroys the CNS depressant activity. 9- Isosteric replacement of oxygen at position 2 by sulphur (2-thiobarbiturates) ⇒↑ CNS depressant activity and shortens the duration of action due to polarity ⇒↑ lipophilicity ⇒ ultrashort acting barbiturates. Barbiturates are classified according to their duration of action into four classes: 1. Long acting barbiturates (> 6 hours) 2. Intermediate acting barbiturates (3-6 hours) 3. Short acting barbiturates (duration 1-3 hours) 4. Ultrashort acting barbiturates (10-30 minutes) 5 1- Long Acting Barbiturates Phenobarbital (Luminal) 5-Ethyl-5-phenylbarbituric acid Phenobarbital is a long acting hypnotic with anticonvulsant activity. It is administered orally or parenterally as the water soluble sodium salt. 2- 3- Intermediate Acting Barbiturates Apobarbital Butalbutal Talbutal Butabarbital Short Acting Barbiturates Cyclobarbital (Phandorn) 5-Ethyl-5-(1-cyclohexenyl)barbituric acid 6 Cyclobarbital is a short acting hypnotic, with 1-cyclohexenyl group at position 5. The normal synthetic route for barbiturates is not applicable for such derivative, it s prepared as follows: 4- Ultrashort Acting Basrbiturates Thiopental Methohexital They are (discussed before) the most lipophlic derivatives (general anesthetics). Metabolism of Barbiturates 1- Oxidation of substituents at carbon 5 by CYP2C19. 2- Aromatic hydroxylation if present. 7 3- Oxidative desulfuration if there is C=S ⇒ C=O (more hydrophilic), e.g. thiopental pentobarbital. 4- N-dealkylation (mephobarbital Phenobarbital). B- Non-barbiturate Sedatives and Hypnotics Many organic compounds of various chemical structures are capable of producing sedation and hypnosis. The major groups of this class are: 1- 1- Acyclic Ureides. 2- Amides and Imides. 3- Alcohols. 4- Aldehydes. Acyclic Ureides (Acyl Ureas) Ureides are the monoacyl derivatives of urea. These derivatives are structurally-related to barbiturates particularly those containing bromine atom at the -carbon of the acyl group. Carbromal Adalin, Bromadal, Nictal: 2-Bromo-2-ethylbutyroylurea 8 Preparation: (Synthesis) 2- Amides and Imides (Piperidinediones) Glutethimide (Doriden) 3-Ethyl-3-phenyl-piperidine-2,6-dione It is structurally related to barbiturates and it is an effective sedative / hypnotic. Synthesis: 9 Methyprylon Noludar, 3,3-diethyl-5-methyl-2,4-piperidinedione These two drugs (piperidinediones) are safer than barbiturates especially in respiratory depression but they cause tolerance and dependence. 3- Alcohols Ethanol has long been known for its sedative effect, the administration of ethanol produce immediate feeling of stimulation followed by depression. Some other alcohols produce weak to moderate sedative and hypnotic activity. Structure-Activity Relationships of Alcohols as CNS Depressants: 1. The hypnotic activity of normal alcohol increases as the molecular weight and lipid solubility increase up to 8 carbons. 2. Branching of the alkyl chain increase the activity. 3. Replacement of hydrogen by a halogen in the alkyl chain greatly increase the activity. Amylene Hydrate (t-Pentanol) 2-Methyl-2-butanol 10 Amylene hydrate is used as mild hypnotic and mainly used as a solvent for tribromoethanol in rectal anesthesia. It is prepared via hydration of Amylene (trimethylethylene). Chlorobutanol Chloretone, Chlorbutol: 1,1,1-Trichloro-2-methyl-2-propanol. 2-Trichloromethyl-2-propanol. Chlorobutanol is used as mild sedative, it is prepared from acetone and chloroform in the presence of solid potassium hydroxide. 11 4- Aldehydes and Their Derivative Chloral Hydrate Notec, Trichloroacetaldehyde monohydrate The CNS depressant activity of chloral hydrate is assumed to be due to its in vivo metabolism to trichloroethanol. It is prepared by prolonged chlorination of ethanol, the final product is crystalline additive compound (Chloral alcoholate), which is collected, distilled with H2SO4 to yield which is mixed with equimolar amount of water to yield chloral hydrate. Paraldehyde Paracetaldehyde, 2,4,6-trimethyl-s-trioxane Paraldehyde is a viscous liquid characterized by strong pungent odour and taste. 12