WG6-Endpoint_Predict..
advertisement

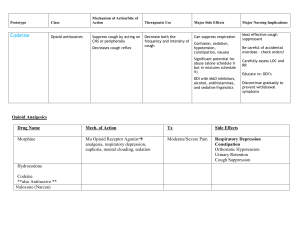
WG6 Nonclinical - Endpoint Predictivity Vision With the requirements for submission of nonclinical data in a standard electronic format (SEND) contained in PDUFA V, we expect that the problem of compilation and exploration of large sets of nonclinical data will become tractable in the near future. We hope to utilize this data in a number of ways, including (but not limited to): Enable better prediction (informing both drug development studies and the clinical protocols and monitoring) Build analysis panels for certain types of concerns Enable better study design (endpoints, timing of collection, etc) Reduce or streamline study endpoints or types based upon evidence Identified Projects/Pilots/Activities The current plan is to develop 3 scientific questions/use cases for piloting/further work. Our high level questions currently are: Use Case 1: Do hERG changes in nonclinical models correlate with x, y or z clinical effects? o Refined: When a significant change in clinical BP (diastolic or systolic) at the maximum effect time point is observed, what is the mean BP change relative to the reference group in dog safety pharmacology studies at the maximum effect time point? Use Case 2: Do transaminase changes in rodents correlate with changes in transaminases in humans? Use Case 3: Are newer kidney biomarkers (e.g. KIM1) more predictive of clinical effects than classic renal injury biomarkers (e.g. creatinine, BUN)? Use Case 4: Do / Which CNS findings in nonclinical species translate to clinical observations? Draft project proposal Use Case 4: Do/Which CNS findings in nonclinical species translate to clinical observations The majority of marketed CNS compounds are basic (containing ionizable nitrogen atom) or neutral. Those compounds are known to interfere with the potassium (e.g.hERG and other ion channels). Quantitatively there relationships between lipophilicity, amphiphilicity and ion channel affinities described, allowing a rough estimate of potential of clinical side effects and an optimization of drug safety profiles at the discovery stage in parallel to biological activity (to be further supported by additional studies). Among others major side effects of CNS compounds include convulsions/seizures[1] and tremors as well as blood pressure and heart rate[2] modulations. Therefore it’s especially of interest for CNS compounds, if CNS findings as convulsion/seizures or tremors in animal studies translate to seizures/convulsions in clinics: Do convulsions/seizures seen in rodent (non -rodent) repeat-dose studies translate to seizures in the clinic? If yes, what is the percentage of time that they translate? Do convulsions in rodent repeat-dose studies translate to other observations in the clinic?” and if preclinical findings for CNS compounds regarding blood pressure (see use case 1) and heart rate modulations translate to clinical findings and are related to specific compound characteristics. Do side effects on heart rate, ECG seen in rodent (non-rodent) repeat-dose studies for CNS compounds can be confirmed in clinics? If yes, what is the percentage of time that they translate? Are there relationships to their overall physicochemical, structural characteristics as for hERG affinity. Literature [1] . Do preclinical seizure models preselect certain adverse effects of antiepileptic drugs. Meldrum, Brian. Epilepsy Research (2002), 50(1-2), 33-40. | Language: English, Database: CAPLUS [2] Effects of Paroxetine and Venlafaxine XR on Heart Rate Variability in Depression. Davidson, Jonathan; Watkins, Lana; Owens, Michael; Krulewicz, Stan; Connor, Kathryn; Carpenter, David; Krishnan, Ranga; Nemeroff, Charles. Journal of Clinical Psychopharmacology (2005), 25(5), 480-484.