eculizumab treatment for severe acute antibody
advertisement

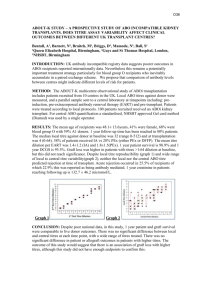
P240 ECULIZUMAB TREATMENT FOR SEVERE ACUTE ANTIBODY-MEDIATED REJECTION (AMR) IN ABO INCOMPATIBLE RENAL TRANSPLANTATION Arunachalam, C¹, Brown, A¹, Talbot, D¹, Sheerin, N¹, Baines, L¹, Carter, V2, Watson, E¹, Scuffell,C¹ ¹Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne, 2Histocompatibility and Immunogenetics, NHSBT Newcastle. Introduction In ABO incompatible (ABOi) renal transplantation, AMR following desensitization protocols usually responds to reinitiation of standard treatment of plasmapheresis (PP), intravenous immunoglobulin (IVIg) and anti-CD20 therapy. However in certain cases of severe AMR characterised by a rapid decline in renal function, oliguria and a concomitant marked rise in donor specific antibodies (DSA), these treatments have been insufficient to rescue the organ. Case report We report a case of severe accelerated AMR treated with Eculizumab (anti-C5 monoclonal antibody). A 57 year old white male with primary disease of IgA nephropathy on haemodialysis was referred for renal transplantation. Previous deceased donor renal transplant which lasted for 16 years was lost due to recurrent disease. He received an ABO and HLA incompatible live donor renal transplant from his son (blood group: donor A1+/recipient O+, 100 HLA mismatch and DSA to HLA-A24 (MFI 3500)). HLAA24 was not a repeat mismatch and he had moderate titres of anti-A antibodies (IgG 1:128, IgM 1:32). Rituximab was administered 1 month before transplantation and Mycophenolate mofetil (MMF) with low dose tacrolimus was begun when pre-operative IA was initiated. IA (Therasorb) was performed 8 times to get the anti-A titres to IgG 1:8, IgM 1:2 on the day of transplantation. IVIg was given after the last IA treatment. Flow and CDC cross matches were negative. Alemtuzumab induction was followed by maintenance immunosuppression with tacrolimus, MMF and prednisolone. The transplant functioned immediately and serum creatinine (sCr) dropped to 69μmol/L on post-operative day (D) 5. He received 4 more sessions of IA (Therasorb) post-transplant. On D6, there was a sharp rise in anti-A titres (IgG 1:32 and IgM 1:32). This was followed by worsening sCr (76 to 158μmol/L), oliguria, drop in platelet count and a LDH rise. USS showed no obstruction with normal blood flow and HLA-DSA remained low. A clinical diagnosis of acute AMR was made. In our experience with three previous failed ABOi transplants, similar rises in antibody titres and deteriorating serum creatinine despite PP/IVIg invariably led to graft thrombosis. This led to the decision to give Eculizumab in this case and in addition he received IVIg and methylprednisolone. Over the following 2 days, urine output improved but renal function continued to deteriorate and anti-A titres continued to rise (both IgG & IgM 1:512). Transplant biopsy was not performed to avoid delay in IA treatment. On D10, he was commenced on Glycosorb IA (selective removal of anti-A antibodies). Glycosorb rather than PP was chosen to avoid Eculizumab removal. Absent CH50 levels throughout the period he was receiving selective IA treatment confirmed that Eculizumab was not removed. On D13 sCr peaked at 694 μmol/L and he needed haemodialysis. Antibody titres stayed high for 5 days even with IA and after 7 sessions of Glycosorb the titres dropped (IgG 1: 16 and IgM 1:8). Graft function recovered with normalization of the platelet count and LDH levels. He received 5 weekly infusions of Eculizumab with no complications and renal function remains stable (sCr 95 μmol/L) at 2 months with minimal proteinuria (urine PCR 23mg/mmol) Conclusion: In the case presented, presumed severe AMR was successfully treated with a combination of Eculizumab and selective antibody removal. Given the very narrow period to intervene for accelerated AMR, Eculizumab may offer a graft-saving treatment option in isolated cases refractory to standard treatment in ABOi kidney transplantation.