AP Chemistry Guided Inquiry Experiments by The College Board
advertisement
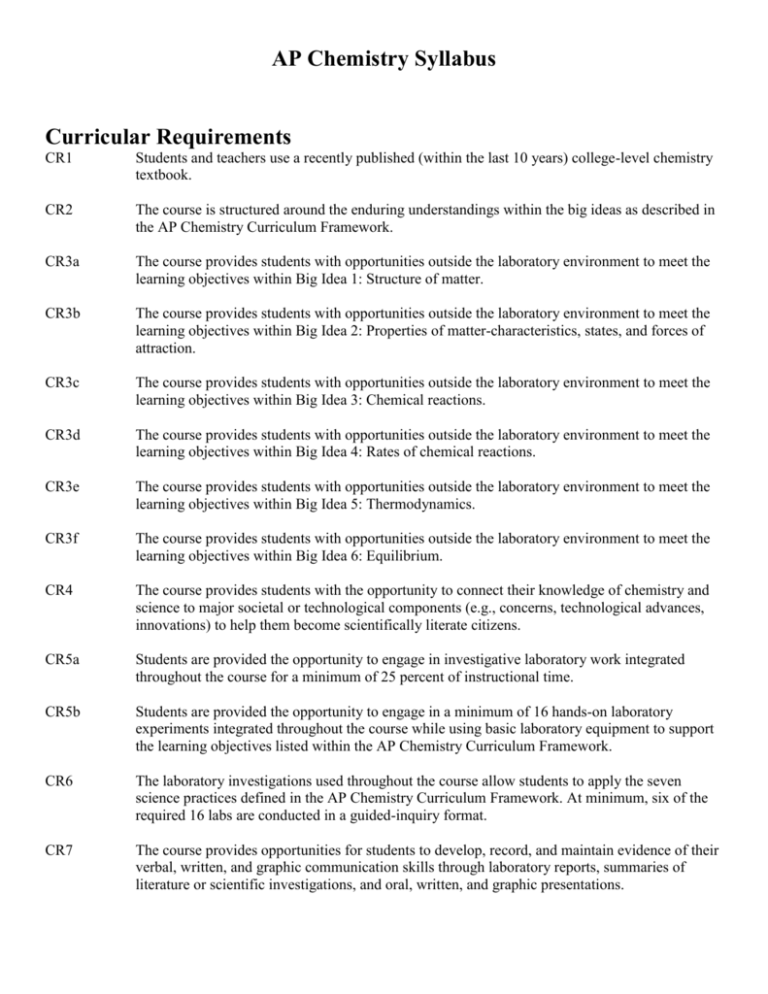
AP Chemistry Syllabus Curricular Requirements CR1 Students and teachers use a recently published (within the last 10 years) college-level chemistry textbook. CR2 The course is structured around the enduring understandings within the big ideas as described in the AP Chemistry Curriculum Framework. CR3a The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 1: Structure of matter. CR3b The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 2: Properties of matter-characteristics, states, and forces of attraction. CR3c The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 3: Chemical reactions. CR3d The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 4: Rates of chemical reactions. CR3e The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 5: Thermodynamics. CR3f The course provides students with opportunities outside the laboratory environment to meet the learning objectives within Big Idea 6: Equilibrium. CR4 The course provides students with the opportunity to connect their knowledge of chemistry and science to major societal or technological components (e.g., concerns, technological advances, innovations) to help them become scientifically literate citizens. CR5a Students are provided the opportunity to engage in investigative laboratory work integrated throughout the course for a minimum of 25 percent of instructional time. CR5b Students are provided the opportunity to engage in a minimum of 16 hands-on laboratory experiments integrated throughout the course while using basic laboratory equipment to support the learning objectives listed within the AP Chemistry Curriculum Framework. CR6 The laboratory investigations used throughout the course allow students to apply the seven science practices defined in the AP Chemistry Curriculum Framework. At minimum, six of the required 16 labs are conducted in a guided-inquiry format. CR7 The course provides opportunities for students to develop, record, and maintain evidence of their verbal, written, and graphic communication skills through laboratory reports, summaries of literature or scientific investigations, and oral, written, and graphic presentations. Course Description The purpose of Advanced Placement Chemistry is to provide a college level course in chemistry and to prepare the student to seek credit and/or appropriate placement in college chemistry courses. This course meets five times per week for one and one-half periods for a total of 75 minutes per day. Laboratory periods average two days per week. Students are engaged in hands-on laboratory work, integrated throughout the course that accounts for more than 25% of the class time. [CR5a] Emphasis is placed on depth of understanding of a topic, rather than breadth of topics. The day before each test is spent in study groups using old AP Chemistry Free Response questions/Study Guides for review. Objectives Students will 1. Learn the inquiry process through numerous laboratory investigations. 2. Gain an understanding of the six big ideas as articulated in the AP Chemistry Curriculum Framework. [CR2] 3. Apply mathematical and scientific knowledge and skills to solve quantitative, qualitative, spatial, and analytic problems. 4. Apply basic arithmetic, algebraic, and geometric concepts. 5. Formulate strategies for the development and testing of hypotheses. 6. Use basic statistical concepts to draw both inferences and conclusions from data. 7. Identify implications and consequences of drawn conclusions. 8. Measure, compare, order, scale, locate, and code accurately. 9. Do scientific research and report and display the results of this research. 10. Learn to think critically in order to solve problems. Textbook, Laboratory Manuals, and Study Guides Chemistry 7th Edition by Zumdahl and Zumdahl, Boston, New York, Houghton Mifflin Company, 2007. [CR1] Fast Track to a Five by Zumdahl and Zumdahl, Evanston, IL, Houghton Mifflin Company, 2006. Laboratory Experiments for Advanced Placement Chemistry by Sally Ann Vonderbrink, Ph.D., Flinn Scientific, Inc., 1995. Multiple Choice and Free Response Questions in Preparation for the AP Chemistry Examination by Demmin and Hostage, 5th Edition, D & S Marketing Systems, 2005. Flinn Labs, Flinn Scientific Company Catalog, 2013. Chemistry: The Central Science Lab Book, by Nelson and Kemp, 10th Edition, Prentice Hall, 2006. The Ultimate Equations Handbook by Hague and Smith, Flinn Scientific, 2001. AP Chemistry Guided Inquiry Experiments by The College Board, 2013. Laboratory Work All of the laboratory experiments in this course are hands-on. Students work individually or in a group of two depending upon the lab. Students collect, process, manipulate, and graph data from both qualitative and quantitative observations. Inquiry is emphasized in many of the experiments that students complete. The laboratory work requires students to design, carry out, and analyze data using guided inquiry principles. For all labs, students are required to report the purpose, procedure, data, data analysis, error analysis, results, and conclusions in a lab report that is submitted for grading. [CR7] All laboratory experiments are intended to be completed in one class period (75 minutes) except guided-inquiry labs that may require two days of work. Laboratory Notebook A laboratory notebook is required for the course. All completed lab reports documenting all lab experiences must be included in the notebook. The notebook is checked every nine weeks with a final check at the end of the course. [CR7] Tests A chapter test is assigned for each chapter. A comprehensive, standardized semester exam is administered at the end of 1st semester and a final exam at the end of the year. AP Exam Review The final ten full class days before the AP Chemistry Exam are used for exam review and practice tests using old AP Chemistry exam materials. Students work in cooperative groups to solve multiple choice and free response problems from previous exams. Students practice net ionic equations and are quizzed on their progress. Several practice AP Exams are administered as part of the two-week review prior to the AP Chemistry Exam. Course Outline [CR2] Chapters in Zumdahl Chemistry AP Chemistry Topic Covered 1. Chemical Foundations None 2. Atoms, Molecules, and Ions Atomic Theory & Atomic Structure (BI 1 & 2) 3. Stoichiometry Stoichiometry (BI 3) 4. Solution Stoichiometry & Chemical Analysis Reaction Types & Stoichiometry (BI 3) 5. Gases Gases (BI 1 & 2) 6. Thermochemistry Thermodynamics (BI 5) 7. Atomic Structure and Periodicity Atomic Theory & Atomic Structure (BI 1 & 2) 8. Bonding -- General Concepts Chemical Bonding (BI 1 & 2) 9. Covalent Bonding: Orbitals Chemical Bonding (BI 1 & 2) 10. Liquids and Solids Liquids & Solids (BI 1 & 2) 11. Properties of Solutions Solutions (BI 2) 12. Chemical Kinetics Kinetics (BI 4) 13. Chemical Equilibrium Equilibrium (BI 6) 14. Acids and Bases Equilibrium (BI 6) 15. Applications of Aqueous Equilibria Equilibrium (BI 6) 16. Spontaneity, Entropy, and Free Energy Thermodynamics (BI 5) 17. Electrochemistry Reaction Types (BI 3) 19. The Rep. Elements: Groups 1A Through 4A Descriptive Chemistry (BI 2) 20. The Rep. Elements: Groups 5A Through 8A Descriptive Chemistry (BI 2) AP Chemistry Exam Review All (BI) refers to Big Ideas. Big Idea 1 – Structure of matter, Big Idea 2 – Properties of matter-characteristics, states and forces of attraction, Big Idea 3 – Chemical reactions, Big Idea 4 – Rates of chemical reactions, Big Idea 5 – Thermodynamics, Big Idea 6 – Equilibrium. Assignments Chapter 1: Read: Problems: Chemical Foundations (6 days) pages 1-30 3-8, 11, 15, 26, 30, 56, 70, 78 Labs: Lab Safety, lab skills, lab preparation Identification of Substances by Physical Properties [CR 5b, 6] (SP 1.4, 6.2; LO 2.15) Separation of the Components of a Mixture [CR 5b, 6] (SP 4.2, 5.1, 6.4; LO 2.10) Activity: Read and interpret information on MSDS sheets for the chapter 1 labs. Determine which substances are soluble in others and the relative densities of the substances. Determine states of matter at room temperature based on MP and BP data. Compare MSDS information to information in the Handbook of Chemistry and Physics. Interpret and explain quantitative measures of solubility. [CR 4] Chapter 2: Read: Problems: Atoms, Molecules and Ions (6 days) pages 39-69 11, 18, 24, 28, 33, 40, 44, 46, 50, 52, 56, 58, 60, 62, 64, 66, 68, 72, 77 Activity: Students are each assigned a substance for which they will measure out 1 mole of the substance. Students will determine the density of their sample using methods practiced in chapter 1 and compare it to the published value. [CR 3b, 4] (SP 6.1; LO 1.1, 1.4) Chapter 3: Read: Problems: Stoichiometry (9 days) pages 77-115 28, 34, 38, 40, 52, 54, 60, 70, 72, 74, 82, 86, 90, 92, 98, 102, 104 Labs: Guided Inquiry: Using the Principles That Each Substance Has Unique Properties to Purify a Mixture: An Experiment Applying Green Chemistry to Purification [CR 5b, 6] (SP 2.1, 4.2, 6.4, 2.2, 5.1, 5.2, 6.1; LO 3.5, 3.3) Guided Inquiry: What Makes Hard Water Hard? [CR 5b, 6] (SP 7.1, 5.1, 2.2, 6.4, 4.2, 5.3, 6.2; LO 1.19, 2.10, 3.2, 3.3) Chemical Reactions of Copper and Percent Yield [CR 5b, 6] (SP 1.4, 2.1, 2.2, 4.2, 5.1, 6.1, 6.4; LO 1.19, 3.2, 3.3, 3.4, 3.10) Activity: Research the term “mass spectroscopy.” Write a summary of the process and how it is used to analyze individual atoms and samples. [CR 3a, 4] (SP 1.4, 1.5; LO 1.14) Determine the percent of water in a hydrate sample given experimental data and the empirical formula of a hydrate and present findings to the class. [CR 3c] (SP 2.2, 6.1; LO 1.3) Chapter 4: Read: Problems: Types of Chemical Reactions and Solution Stoichiometry (8 days) pages 127-170 9, 10, 17, 19, 22, 24, 26, 28, 30, 32, 36, 38, 40, 44, 46, 48, 50, 56, 58, 60, 64, 68, 72, 74 Labs: Guided Inquiry: How Can We Determine the Actual Percentage of H2O2 in a Drugstore Bottle of Hydrogen Peroxide? [CR 5b, 6] (SP 4.2, 5.1, 6.4, 2.1, 6.1, 2.2; LO 3.9, 1.2, 3.3) Oxidation Reduction Titrations Using KMnO4 [CR 5b, 6] (SP 2.2, 5.1, 6.4, 4.2; LO 3.3, 1.20) Chapter 5: Read: Problems: Gases (8 days) pages 179-216 20, 31, 34, 36, 42, 46, 52, 56, 62, 66, 67, 72, 76, 80, 82, 84, 88 Labs: Determining the Molar Volume of a Gas [CR 5b, 6] (SP 1.4, 6.4, 1.3, 7.2, 2.2, 2.3; LO 2.4, 2.5, 2.6) Calcualtion of the Molar Mass of a Gas [CR 5b, 6] (SP 1.4, 6.4, 1.3, 7.2, 2.2, 2.3; LO 2.4, 2.5, 2.6) Activity: Read the article on the top of page 197. Look up/determine the number and type of airbags in the car that you most often drive. Assuming that each airbag must inflate to about 35 L, how many grams of sodium azide are in your car, ready in the case of a car crash? Assume 1 atm and 20°C. Write a summary of your findings. [CR 3c, 4] (SP 1.4, 6.2; LO 2.4) Chapter 6: Read: Problems: Thermochemistry (8 days) pages 229-265 10, 12, 22, 26, 32, 34, 36, 38, 42a, c, 44, 46, 52, 56, 58, 60, 62, 64, 68b, c, 82 Labs: Guided Inquiry: The Hand Warmer Design Challenge: Where does the Heat Come From? [5b, 6] (SP 1.4, 6.4, 7.2, 4.2, 5.3, 2.2, 2.3, 5.1; LO 5.7, 5.6) Exothermic and Endothermic Reactions [CR 5b, 6] (SP 2.2, 2.3, 4.2, 5.1, 6.4, 7.2; LO 5.6, 5.7) Chapter 7: Read: Problems: Atomic Structure and Periodicity (9 days) pages 275-320 18, 26, 36, 38, 46, 58, 60, 62, 66, 72, 74, 78, 84, 86, 96, 109, 110 Labs: Flame Tests and Solution Colors [CR 4, 5b, 6] (SP 5.3; LO 1.13, 1.12) Guided Inquiry: What is the Relationship Between the Concentration of a Solution and the Amount of Light Transmitted through the Solution? [CR 5b, 6] (SP 2.2, 5.1, 4.2, 4.1, 6.4; LO 1.15, 1.16) Activity: Students are given pairs of elements are asked to rationalize the differences in electronegativity, radius, etc. based on electron arrangement. Findings are presented to the class. [CR 3a] (SP 6.4, 1.5, 6.2; LO 1.9, 1.5) Chapter 8: Read: Problems: Bonding and General Concepts (6 days) pages 329-381 14, 18, 20, 22, 24, 26, 29, 30, 36, 38, 52, 54, 68, 74, 75, 78, 80, 84, 86, 92, 96, 103 Activity: Given a formula, students build molecular models and draw structural formulas for each. Identify geometry, bond angles, polar bonds and dipole moments for each. [CR 3a, b] (SP 1.4, 1.1; LO 2.21, 2.23) Chapter 9: Read: Problems: Covalent Bonding: Orbitals (6 days) pages 391-403 8, 10, 14, 16, 30, 34 Lab: Guided Inquiry: How Can Color be used to Determine the Mass Percent of Copper in Brass? [CR 5b, 6] (SP 4.2, 5.1, 2.2, 6.4; LO1.16, 3.4) Chapter 10: Liquids and Solids (8 days) Read: 425-436, 440-474 Problems: 16, 20, 28, 32, 34, 36, 38, 39, 72, 80, 82, 84, 88, 91 Labs: Guided Inquiry: What’s in that Bottle? [CR 5b, 6] (SP 4.2, 6.4, 1.1, 6.2, 7.1; LO 2.22, 2.24, 2.28, 2.32) Properties of Solids [CR 5b, 6] (SP 1.4, 6.2, 6.4, 1.1, 7.1; LO 2.15, 2.14, 2.19, 2.20) Activity: Given a phase diagram graph for water, explain the significance of the negative slope of the line between solid and liquid phases. Compare water’s phase diagrams to other substances. Interpret boiling point and melting point at standard pressure. [CR 4] (LO 2.16) Read the “Chemical Impact” articles in chapter 10 of the text. Look up additional information on one of the topics and write a two page typed summary of your findings. [CR 4] Chapter 11: Properties of Solutions (7 days) Read: pages 285-518 Problems: 12, 14, 16, 20, 24, 25, 28, 32, 36, 38, 44, 46, 48, 54, 58, 60, 70 Lab: Molar Mass by Freezing Point Depression [CR 5b, 6] (SP 1.1, 1.4, 7.1; LO 2.19) Activity: How is freezing point depression related to road salt and ice cream making? Research this question and write a response. Discuss answers as a class. [CR 3b, 4] Chapter 12: Chemical Kinetics Read: pages 527-566 Problems: 10, 16, 20, 26, 28, 31, 33, 35, 37, 41, 43, 47, 49, 51, 53, 55, 59, 65 Labs: Guided Inquiry: What is the Rate law of the Fading of Crystal Violet Using Beers Law? [CR 5b, 6] (SP 5.1, 6.4, 4.2, 5.1; LO 4.1, 4.2) Kinetics of a Reaction [CR 5b, 6] (SP 5.1, 6.4, 4.2, 5.1; LO 4.1, 4.2) Activity: Demonstrate and discuss “rate determining” by passing papers from one student to the next across the room. Why do chemical reactions not necessarily happen in “one step?” [CR 3d, 4] (SP 4.2, 5.1; LO 4.1) Research biological enzymes. Why are they necessary? What is their function? What would happen to chemical reactions in the body if there were not present? [CR 3d, 4] (SP 1.5; LO 4.8) Students create energy diagrams to explain why catalysts or raising the temperature can increase the rate of a chemical reaction [CR3d] (SP 1.5; LO 4.8) Chapter 13: Chemical Equilibrium (12 days) Read: pages 578-612 Problems: 13, 17, 18, 20, 22, 26, 28, 30, 32, 36, 38, 40, 44, 46, 48, 54, 58, 64, 67, 74 Labs: The Determination of Keq for FeSCN+2 [CR 5b, 6] SP 2.2, 6.4; LO 6.5, 6.6) Guided Inquiry: Can We Make the Colors of the Rainbow? An Application of LeChatelier’s Principle [CR 5b, 6] (SP 4.2; LO 6.9) Activity: Research the history of the development of the Haber Process. Who was Fritz Haber? Why was the development of the process significant? What affect did it have on the war? What happened to Haber? [CR 3f] Students create a model and description of the term “dynamic equilibrium.” Present models and description to the class. [CR 3f] Chapter 14: Acids and Bases (12 days) Read: pages 623-672 Problems: 17, 25, 28, 30, 32, 34, 38, 40, 42, 44, 48, 52, 54, 56, 60, 64, 66, 70, 72, 76, 78, 84, 88, 98, 102, 104, 112, 114, 118, 124 Labs: Determination of the Ka of a Weak Acid [CR 5b, 6] (SP 1.1, 1.4, 2.3, 6.4; LO 6.11) Hydrolysis – Acidic, Basic, or Neutral? [CR 5b, 6] (SP 1.1, 1.4, 2.3, 6.4; LO 6.11) Chapter 15: Application of Aqueous Equilibria (13 days) Read: Pages 681-739 Problem: 24, 26, 43, 40, 44, 46, 48, 52, 56, 62, 66, 76, 78, 80, 86, 92, 98, 100 Labs: Acid-Base Titrations [CR 5b, 6] (SP 5.1, 6.4; LO 6.13) Determination of a Solubility Product Constant for a Sparingly Soluble Salt [CR 5b, 6] (SP 2.2, 2.3, 6.4; LO 6.21, 6.22) Guided Inquiry: To What Extent do Common Household Products Have Buffering Activity? [CR 5b, 6] (SP 6.4, 4.2, 5.1, 4.3, 5.3, 6.2; LO 6.20, 1.20) Chapter 16: Spontaneity, Entropy and Free Energy (8 days) Read: Pages 749-782 Problems: 18, 20, 24, 26, 28, 30, 32, 34, 36, 38, 44, 54, 58, 60, 62, 70 Activity: Using the PHET simulations, open “Reversible Reactions.” Make adjustments to numbers of particles, temperature, and shape of the graph. Make observations and compile a list as a class of cause-effect relationships for reactions based on the simulation. [CR 3e] Chapter 17: Electrochemistry (10 days) Read: pages 791-829 Problems: 17, 21, 26, 28, 30, 32, 36, 56, 58, 64, 72, 76, 80, 84, 95 Labs: An Activity Series [CR 5b, 6] (SP 1.4, 6.1; LO 3.9, 3.10) Oxidation – Reduction Titrations [CR 5b, 6] (SP 4.2, 5.1; LO 3.9) Chapter 19: The Representative Elements: Groups 1A through 4A (4 days) Read: Pages 875-895 Problems: 2, 8, 10, 16, 18, 19, 22, 24, 26, 28, 30, 32, 34, 36, 39, 42, 44, 46, 48 Lab: Gravimetric Analysis of a Chloride Salt [CR 5b, 6] (SP 4.2, 5.1, 6.4; LO 1.19) Activity: Read the article on page 893. Research and the history of and current issues related to lead and mercury poisoning. [CR 4] Chapter 20: The Representative Elements: Groups 5A through 8A (3 days) Read: pages 901-935 Problems: 2, 10, 12, 14, 20, 22, 25, 30, 33, 38