pat3556-sup-0001-documentS1
advertisement

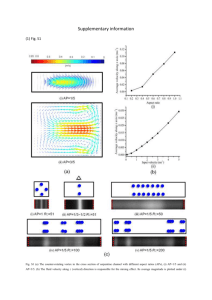
Supplementary information Poly(2-oxazoline) block copolymer based formulations of taxanes: Effect of copolymer and drug structure, concentration and environmental factors Youngee Seoa, Anita Schulzb,#, Yingchao Hanc,¶, Zhijian Hea, Herdis Bludaub, Xiaomeng Wana, Jing Tongc, Tatiana K. Bronichc, Marina Sokolskya, Robert Luxenhoferd,*, Rainer Jordanb,*, Alexander V. Kabanova,e,* a. Center for Nanotechnology in Drug Delivery, Division of Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7362, United States b. Professur für Makromolekulare Chemie, Department Chemie, Technische Universität Dresden, Zellescher Weg 19, 01069 Dresden, Germany c. Center for Drug Delivery and Nanomedicine and Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE 68198-5830, United States d Functional Polymer Materials, Chair of Advanced Materials Synthesis, Department Chemistry and Pharmacy, Universität Würzburg,Rontgenring 11, 97070 Würzburg, Germany e. Laboratory of Chemical Design of Bionanomaterials, Faculty of Chemistry, M.V. Lomonosov Moscow State University, Moscow 119992, Russia # Present address: A. S.: Institute des Biomolécules Max Mousseron (IBMM), UMR CNRS 5247, University of Montpellier, Faculty of Pharmacy, 15 Av. C. Flahault, Montpellier, 34093, France Present address: State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, P.R. China ¶ 1 Supplementary Materials and Methods Polymerization of Homopolymers The initiator MeOTf and the monomer were dissolved in acetonitrile (ACN)/chlorobenzene (1/1, v/v) at room temperature (RT) under dry and inert conditions. The reaction mixture was irradiated in the microwave at 150 W and 90 °C for 1h. Termination was carried out with 3 eq with respect to the initiator of 1-BOC-piperazine (BOC-Pip) dissolved in ACN (250 g/L) for 1h, 50 W and 40 °C. An excess of potassium carbonate was added and the mixture was stirred over night. After filtration the solvent was removed. H1 was dissolved in ACN and precipitated in water. H2 and H3 were dissolved in methanol (MeOH) and precipitated in water. H4 was dissolved in MeOH/ dichloromethane (DCM) (1/1; v/v) and precipitated in water. H5 was dissolved in DCM and precipitated in diethyl ether. The results of the analytical characterization of the homopolymers are summarized in Table S2. Drop shape analysis The homopolymers were dissolved in a volatile organic solvent at concentrations between 1-10 g/L. The polymer solution was deposited on a piece of a silicon wafer. Depending on the hydrophobicity of the polymer, the silicon wafer was hydrophobized with a 5% (v/v) dichlorodimethylsilane solution in DCM prior polymer deposition. Three to six contact angles were measured depending on the available surface covered with polymer. The data are presented as means±standard deviation (SD). Transmission Electron Microscopy (TEM) A drop of the blank micelles (polymer concentration 100 ug/mL) was placed onto carbon Type-B 200 mesh, cupper grid (Ted Pella). The negatively stained samples were prepared by 3 % uranyl acetate solution. Excess solution was removed by filter paper. Specimens were examined with JEOL 2010F-FasTEM (JEOL USA, Peabody, MA, USA), operating at 200 kV. 2 Supplementary Figures Fig. S1. TEM images of the empty D1 micelles. Fig. S2. Effect of DTX feed concentration on the (a) solubilization of DTX in T1 micelles in DI water at RT as represented by DC, LE and LC values and (b) z-averaged particle size and PDI. Concentration of T1 was set at 10 g/L in both experiments and DTX concentration was varied from 1 to 10 g/L. Data are mean ± SD (n = 3). Fig. S3. Effect of T1 and DTX feed concentrations on the (a) solubilization of DTX in T1 as represented by DC, LE and LC values, and (b) z-averaged particle size and PDI. T1 concentration was varied from 1 to 50 g/L at constant POx/DTX weight ratio of 1:1. Data are mean ± SD (n = 3). 3 Fig. S4. Effect of DTX feed concentration on the (a) solubilization of DTX in T8 as represented by DC, LE and LC values, and (b) z-averaged particle size and PDI. Concentration of T8 was set at 10 g/L in both experiments and DTX concentration was varied from 1 to 10 g/L. Data are mean ± SD (n = 3). Fig. S5. Effect of T8 and DTX feed concentrations on the (a) solubilization of DTX in T8 as represented by DC, LE and LC values and (b) z-averaged particle size and PDI. T8 concentration was varied from 1 to 50 g/L at constant POx/DTX weight ratio of 1:0.6. Data are mean ± SD (n = 3). Fig. S6. Effect of storage temperature on the stability of 50 g/L T1 micellar formulation of (a) PTX and (b) DTX (drug feed concentration 50 g/L) in DI water. 4 Fig. S7. Effect of dilution on the stability of 50 g/L T1 micellar formulations of PTX in DI water. PTX feed concentration was 50 g/L. Fig. S8. Colloidal stability (size and PDI) of diluted T1 micelle formulations of PTX in DI water, 10 mM NaCl and PBS. The formulations were diluted 50 fold (a); 100 fold (b); 200 fold (c); 400 fold (d) and 500 fold (e,f). The formulations were prepared at 50 g/L T1 and 50 g/L PTX feed concentration and stored for 2 weeks at 250C. *p<0.05; **p<0.01 5 Fig. S9. Effect of time on the content of PTX (% of initial amount) after 500-fold dilution of T1 micelles. The formulations were prepared at 50 g/L T1 and 50 g/L PTX feed concentration. *p<0.05; **p<0.01 6 Supplementary Tables Table S1 Reported copolymers used for solubilizing taxanes Copolymers Poly(N-vinylpyrrolidone)-b-poly( D,L-lactide) [1] (PVP-b-PDLLA) Poly(N-(2-hydroxypropyl) methacrylamide lactate)-b-poly(ethylene glycol) [2] (pHPMAmDL-b-PEG) Poly(2-ethyl-2-oxazoline)-b-poly(e-caprolactone) [3] Poly(ethylene oxide)-b-poly(styrene oxide) [4] Poly(ethylene oxide)-b-poly(butylene oxide) Poly(ε-caprolactone)-b-monomethoxyl poly-(ethylene glycol) [5] Poly(ethylene oxide)-b-poly(epsiloncaprolactone) [6] (PEO-PCL) Poly(ethylene glycol)-block-poly(D,L lactic acid) [7] (PEG-b-PDLLA) Polyethylene glycol-b-polyaspartate [8] (PEG-b-PAsp) Methoxy-poly(ethyleneglycol)-b-oligo(ecaprolactone) [9] (mPEG-b-OCL) Poly(N-isopropylacrylamide-co-acrylamide)-b-poly(DL-lactide) (poly(IPAAm-co-AAm)-b-PDLLA) [10] Polyethylene glycol-cholic acid telodendrimer [11] (PEG5k-CA8) * Maximal LC * PTX 4.5% DTX 3.7% Diameter 56.0 nm 22.0 nm PTX 9.6% 60 nm PTX 7.1% 18.3-23.4 nm DTX 4.0% 21 nm PTX 5.1% 161.1 nm DTX 8.3% 50 nm PTX 11.8% DTX 11.2% 38.8 nm 37.3 nm PTX 18.7% 85 nm PTX 32.0% DTX 16.7% 23-200 nm (PDI>0.2) 16 nm (PDI<0.1) DTX 21.3% 80 nm PTX 25.9% 90 nm Re-computed using equation (1) 7 Table S2. Analytical data of synthesized homopolymers. ID Structure Yield [%] NBoc H1 N N O 24 H2 72 3.3 3.3 4.6 1.01 1.06 54±1 56 4.0 3.1 4.5 1.03 1.06 63±2 28 3.6 3.0 4.4 1.06 1.05 81±2 24 3.7 3.5 4.8 1.02 1.06 85±2 15 5.8 4.5 5.2 1.02 1.06 93±2 NBoc N N O H3 30 NBoc N N O H4 27 NBoc N N O H5 MNMR MMALDI MGPC ƉMALDI ƉGPC Contact [kg/mol] [kg/mol] [kg/mol] angle H2O [degree] 25 NBoc N N O 33 8 Table S3. Size of 50 g/L T1 micelle PTX formulations (PTX feed concentration 50 g/L) before and after lyophilization and redispersion Size (nm) PDI Before lyophilization DI water 28.6±1.1 0.14±0.01 Immediately after redispersion DI water PBS 26.3±2.0 34.2±1.3 0.27±0.06 0.07±0.01 2 days after redispersion DI water PBS 29.2±0.6 36.9±2.4 0.14±0.06 0.11±0.08 9 Table S4. IC50 values of T1 micellar PTX in different cell lines by MTT assaya. Formulation Free PTX Micellar PTX Free DTX Micellar DTX MCF-7 2.0 ±0.40 1.5 ±0.57 0.64 ±0.14 4.8 ±0.71** IC50 values (nM) MDA-MB-231 0.47 ± 0.12 0.39 ± 0.13 3.2 ± 1.8 1.6 ± 0.76 PC3 0.62±0.36 0.37±0.07 1.5±0.48 0.49±0.47 a IC50 are calculated by Graphpad software from 3 independent experiments. Data are mean ± SEM. Representative experiments are shown in Fig. 11. ** p<0.01 compared to free DTX. 10 Table S5. IC50 values of PTX-POx micellar drugs against cancer cells by MTT assaya Formulation T1 T8 D1 MCF-7 1.1 ±0.22 0.42 ±0.22 0.37 ±0.11 MDA-MB-231 0.35 ± 0.18 0.51 ± 0.16 0.53 ± 0.12 IC50 values (nM) PC3 LCC-6 0.53±0.25 37 ± 11 0.61±0.13 35±9.6 0.83±0.22 11±3.4 LCC-6-MDR 3460±600** 10200±1900** 8720±2070** a IC50 are calculated by Graphpad software from independent experiments. Data are mean ± SEM. Representative experiments are shown in Fig. 12. ** p<0.01 compared to LCC-6-wild type group. 11 Supplementary References [1] D. Le Garrec, S. Gori, L. Luo, D. Lessard, D. C. Smith, M.-A. Yessine, M. Ranger, J.-C. Lerous, J. Control. Release 2004, 99, 83. [2] O. Soga, C. F. van Nostrum, Fens Marcel, C. J. F. Rijcken, R. M. Schiffelers, G. Storm, W. E. Hennink, J. Control. Release 2005, 103, 341. [3] S. C. Lee, C. Kim, I. C. Kwon, H. Chung, S. Y. Jeong, J. Control. Release 2003, 89, 437.. [4] M. Elsabahy, M.-È. Perron, N. Bertrand, G.-e. Yu, J.-C. Leroux, Biomacromolecules 2007, 8, 2250. [5] X. Shuai, T. Merdan, A. K. Schaper, F. Xi, T. Kissel, Bioconjug. Chem. 2004, 15, 441. [6] L. Ostacolo, M. Marra, F. Ungaro, S. Zappavigna, G. Maglio, F. Quaglia, A. Abbruzzese, M. Caraglia, J. Control. Release 2010, 148, 255. [7] H.-C. Shin, A. W. Alani, D. A. Rao, N. C. Rockich, G. S. Kwon, J. Control. Release 2009, 140, 294. [8] T. Hamaguchi, Y. Matsumura, M. Suzuki, K. Shimizu, R. Goda, I. Nakamura, I. Nakatomi, M. Yokoyama, K. Kataoka, T. Kakizoe, Br. J. cancer 2005, 92, 1240. [9] M. G. Carstens, P. H. de Jong, C. F. van Nostrum, J. Kemmink, R. Verrijk, L. G. De Leede, D. J. Crommelin, W. E. Hennink, Eur. J. Pharm. Biopharm. 2008, 68, 596. [10] B. Liu, M. Yang, R. Li, Y. Ding, X. Qian, L. Yu, X. Jiang, Eur. J. Pharm. Biopharm. 2008, 69, 527. [11] K. Xiao, J. Luo, W. L. Fowler, Y. Li, J. S. Lee, L. Xing, R. H. Cheng, L. Wang, K. S. Lam, Biomaterials 2009, 30, 6006. 12