Corrected_Supplimentary file_L12-06699R_26
advertisement

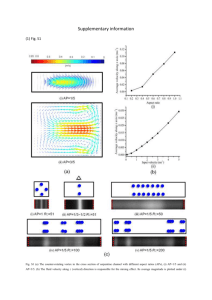
EPAPS Document No.: To be filled in by AIP staff]: [to be filled in by AIP staff] All Authors: Surendra K. Yadav, Jay Singh, Ved V. Agrawal and B. D. Malhotra Title: Nanostructured nickel oxide film for application to fish freshness biosensor Description: Fig [S1] Scheme Fig [S2] Hall–Williamson plot Fig [S3] FTIR Fig [S4] SEM Fig [S5] Contac angle measurement Fig [S6] EIS Fig [S7] Scan rate Fig [S8] Response time Fig [S9] Shelf-life Fig [S10] Real sample analysis Table I Total No. of Files: 1 File Names: README.TXT, and supplementary file File Types: Doc Special Instructions: None Contact Information: Prof. B.D. Malhotra, Department of Biotechnology, Delhi Technological University, Shahbad Daulatpur, Delhi 110042, India. Email: bansi.malhotra@gmail.com, Tel.: +91-11-27294668 Fax: 91-11-27871023 Supplementary File Fig [S1] Schematic representation of xanthine biosensor and mechanism of electron exchange between xanthine oxidase and electrode surface. cos Fig [S2] Hall–Williamson plot of the n-NiO nanoparticles. 2.8x10 -3 2.6x10 -3 2.4x10 -3 2.2x10 -3 2.0x10 -3 1.8x10 -3 1.6x10 -3 1.4x10 -3 1.2x10 -3 Slope of the Curve = - 3.46x10 -3 0.60 0.65 0.70 0.75 0.80 0.85 0.90 0.95 1.00 Sin Fig [S3] Fourier Transform Infrared spectra of (a) n-NiO/ITO electrode and (b) XOx/nNiO/ITO bioelectrode. 400 3462 1630 682 462 Transmittance(a.u.) 350 300 2581 2359 1700 2208 1377 2230 1830 250 200 150 682 780 3090 b a 3746 100 940 1169 50 4000 3500 3000 2500 2000 -1 Wavenumber (cm ) 1500 1000 500 Fig [S4] Scanning electron microscopic images of n-NiO nanoparticle (a & b) and XOx/nNiO/ITO (c & d) bioelectrode at different magnification. (a) (b) (c) (d) Fig [S5] Contact Angle measurement of ITO electrode (i), n-NiO nanoparticle (ii) and XOx/n-NiO/ITO bioelectrodes (iii) CA 1200 (i) CA 980 (ii) CA 680 (iii) Fig [S6] The Nyquist plot of the electrochemical impedance spectra of (a) ITO, (b) nNiO/ITO electrode (c) XOx/n-NiO/ITO bioelectrode having phosphate buffer (50mM, pH 7.0, 0.9% NaCl) containing 5mM [Fe(CN)6]3−/4−. Fig [S7] Electrochemical response curve potential V/S current as a function of scan rate inset current vs (scan rate)1/2. Fig [S8] Electro chemical response time on XOx/n-NiO/ITO bioelectrode from 5 to 40 seconds of incubation period. -5 8.9x10 -5 8.8x10 -5 8.7x10 Current (A) -5 8.6x10 -5 8.5x10 -5 8.4x10 -5 8.3x10 -5 8.2x10 -5 8.1x10 -5 8.0x10 0 5 10 15 20 25 30 Response Time (s) 35 40 45 Fig [S9] Self life curve for XOx/n-NiO/ITO bioelectrode as a function of time. Current (A) Fig [S10] Linearity curve for real sample (fish) analysis for xanthine estimation. 2.0x10 -4 1.5x10 -4 1.0x10 -4 5.0x10 -5 0.0 -5.0x10 -5 -1.0x10 -4 -1.5x10 -4 -2.0x10 -4 1 2 3 4 Days 5 6 7 Table I. Characteristics of the xanthine oxidase based biosensor including those reported in literature. Material Detection Linearity Sensitivity Response Storage (%) limit (μM) (μM) (µA/µM-cm2) time(s) Time(days) Loss Ref. Graphite 1.5 1.5-70 0.39 60 ------ ------ 1 Graphite rod 0.1 0.1-0.6 ------ 35 15 ------ 2 Polypyrrole 1 1-20 ------ ------ ------ ------ 3 Zinc oxide-PPY 0.8 0.8–40 ------ 5 100 40 4 Nafion 0.5 2-185 ------ 30 10 ------ 5 2 2–50 ------ 150 -------- ------ 6 Au-polypyrrole 0.4 0.4 - 100 ------ ------ 100 40 7 Modified graphite 4.5 0-40 0.210 120 500 h 35 8 (c-MWCNT)(PANI) 0.6 0.6–58 ------ ------ 100 50 9 ------ 1 - 100 ------ 150 ------ ------ 10 CNT glassy carbon 0.1 0.2 - 10 ------ ------ 15 ----- 11 Glassy carbon paste 5.3 20-80 ------ ------ ------ ------ 12 ZnO/Ch/MWCNT/PANI 0.1 0.1–100 ------ 4 30 30 13 n-NiO Nanoparticle 2.9 10-200 0.6214 15 100 30 Present DW-CNT MWCNT Work. References 1. T. Dodevska, E. Horozova, N. Dimcheva, Cent. Eur. J. Chem. 8, 19 (2010). 2. R. Devi, J. Narang, S. Yadav, and C. S. Pundir, J. Anal Chem. 67, 273(2012). 3. Y. Liu, L. Nie, W. Tao, S. Yao, Electroanalysis, 16, 1271(2004). 4. R. Devi, M. Thakur, C.S. Pundir, Biosens. Bioelectron. 26, 3420 (2011). 5. H. S. Nakatani, L. V. d.santos, C. P. Pelegrine, S. T.M. Gomes, M. Matsushita, N. E. de Souza, J. V. Visentainer , Am. J. Biochem. Biotech.1, 85(2005). 6. U. anik, S. Çevik, Microchim Acta, 166, 209 (2009). 7. R. Devi, S. Yadav, C.S. Pundir, Coll. and Sur. A: Physicochem. Eng. Asp. 394, 38(2012). 8. N. Dimcheva, E. Horozova, Z. Jordanova, Z. Naturforsch. 57c, 883 (2002). 9. R. Devi, S. Yadav, C.S. Pundir, Biochem. Eng. J. 58, 148 (2011). 10. U. anik, M. Cubukcu, Turk J Chem. 32, 711(2008). 11. Y. Gao, C. Shen, J. Di, Y. Tu, Mat. Sci. and Eng. C 29, 2213 (2009). 12. U. A. Kirgoz, S. Timur, J. Wang, A. Telefoncu, Electrochem. Comm. 6, 913(2004). 13. R. Devi, S. Yadav, C.S. Pundir, Analyst. 137, 754 (2012)