Chemistry
advertisement

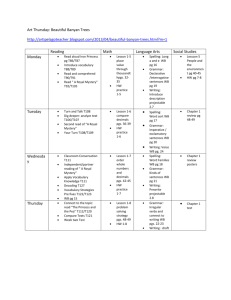
Physical Science Unit Map ____________________________________________________________________________________________________________ Unit of Instruction: Chemistry Learning Target **Pre-Lesson Material** Resource/Text Section/Packet Pages Holt – Physical Science: Chapters 2 – 3 Physical Science: Matter Packet (MP) Learning Activities Pre- Lesson Key Words Chemistry Matter Assessment Notes States of Matter Study Guide: MP pgs 7 – 8 Density problems: MP pg 9a – 9b Lab 1: Chromatography: MP pgs10 – 11 Lab 2: Investigating Chemical Reactions: MP pgs 12 – 13 Lab 3: What are those substances? : MP pgs 14 – 15 Matter Packet Review: MP pgs 16 – 17 Video: Elements Discover : MP pgs 18 – 19 Element Atom Compound Molecule Unit Exam Chemical formula Reactivity Pure substance Mixture Melting point Boiling point Density Flammability Physical change Plasma Energy Thermal energy Evaporation Sublimation Chemical change Condensation Fluid Buoyant force Pressure Archimedes’ principle Gay-Lussac’s Law Pascal Pascal’s principle Viscosity Boyle’s Law Charles’s Law Physical Science Unit Map ____________________________________________________________________________________________________________ Learning Target 1. I can describe the relative charges of an electron, proton, and neutron Resource/Text Section/Packet Pages Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) 2. I can describe the relative masses of an electron, proton, and neutron 3. I can describe the relative locations of an electron, proton, and neutron Learning Activities Assessment Notes H1) Development of Atomic Theory: CP pg 1 H2) What is an Atom: CP pg 2 H3) Atomic Structure: CP pgs 3 – 4 H6) Atomic Math Challenge: CP pgs 8 – 9 H7) Atoms, Elements and the Periodic Table: CP pgs 10 – 11 Unit Exam Periodic Table Project Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes H1) Development of Atomic Theory: CP pg 1 H2) What is an Atom: CP pg 2 H3) Atomic Structure: CP pgs 3 – 4 H6) Atomic Math Challenge: CP pgs 8 – 9 H7) Atoms, Elements and the Periodic Table: CP pgs 10 – 11 Unit Exam Periodic Table Project Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes H1) Development of Atomic Theory: CP pg 1 H2) What is an Atom: CP pg 2 H3) Atomic Structure: CP pgs 3 – 4 H6) Atomic Math Challenge: CP pgs 8 – 9 H7) Atoms, Elements and the Periodic Table: CP pgs 10 – 11 H8) Periodic trends and Groups: CP pgs 20 – 21 H9) Electron dot diagrams: CP pgs 14 – 15 Unit Exam Periodic Table Project Physical Science Unit Map ____________________________________________________________________________________________________________ 4. I can explain how elements are arranged in the periodic table Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) 5. I can explain the relationship among elements in the same column Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) 6. I can explain how atoms are rearranged in a chemical reaction Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes H4) Gold Dust Kid H5) Chemistry Pun’s H7) Atoms, Elements and the Periodic Table: CP pgs 10 – 11 H8) Periodic trends and Groups: CP pgs 20 – 21 H10) Periodic Table Basics: CP pgs 16 – 19 Unit Exam Periodic Table Project Notes H4) Gold Dust Kid H5) Chemistry Pun’s H7) Atoms, Elements and the Periodic Table: CP pgs 10 – 11 H8) Periodic trends and Groups: CP pgs 20 – 21 H9) Electron dot diagrams: CP pgs 14 – 15 H10) Periodic Table Basics: CP pgs 16 – 19 Unit Exam Periodic Table Project Notes H10) Periodic Table Basics: CP pgs 16 – 19 H11) Bonding Basics Ionic: CP pgs 22 – 24 H12) Formulas: CP pgs 25 – 27 H13) Polyatomic Ions: CP pg 23 H14) Bonding Basics: Covalent: CP pgs 29 – 30 H15) Bonding Practice: CP pages 31 – 32 H16) Balancing Act: CP pages 34 – 42 Unit Exam Physical Science Unit Map ____________________________________________________________________________________________________________ 7. I can explain how a chemical reaction illustrates the law of conservation of mass 8. I can describe a chemical reaction using words 9. I can describe a chemical reaction using symbolic equations Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes Lab 1: Conservation of Mass Unit Exam Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes H12) Formulas: CP pgs 25 – 27 H15) Bonding Practice: CP pages 31 – 32 H16) Balancing Act: CP pages 34 – 42 Unit Exam Holt – Physical Science: Chapters 4 – 6 Physical Science: Chemistry Packet (CP) Notes H12) Formulas: CP pgs 25 – 27 H15) Bonding Practice: CP pages 31 – 32 H16) Balancing Act: CP pages 34 – 42 Unit Exam Physical Science Unit Map ____________________________________________________________________________________________________________ Key Words Nucleus Proton Neutron Electron Orbital Valence electron Periodic law Period Group Ion Atomic number Mass number Isotope Atomic mass unit (amu) Alkaline-earth metal Molar mass Average atomic mass Transition metal Metal Nonmetal Semiconductor Halogen Noble gas Mole Conversion factor Metallic bond Chemical bond Bond length Covalent bond Chemical structure Polyatomic ion Alkali metal Avagadro’s constant Bond angle Molecular formula Reactant Organic compound Product Polymer Carbohydrate Protein Empirical formula Amino acid Chemical energy Decomposition reaction Electrolysis Combustion reaction Radical Chemical equation Mole ratio Exothermic reaction Singledisplacement reaction Catalyst Endothermic reaction Doubledisplacement reaction Enzyme Synthesis reaction Oxidationreduction reaction Substrate Chemical equilibrium Ionic bond Physical Science Unit Map ____________________________________________________________________________________________________________ Assessment Plan Notes Target Target Target Target Target Target Target Target Target 1 2 3 4 5 6 7 8 9 X X X X X X X X X 1 X X X 2 X X X 3 X X X 4 X X 5 X X Homework Assignments Lab 1 Periodic Table Project 6 7 8 9 10 11 12 13 14 15 16 X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Unit Exam X X X X X X X X X