CP Chemistry
advertisement
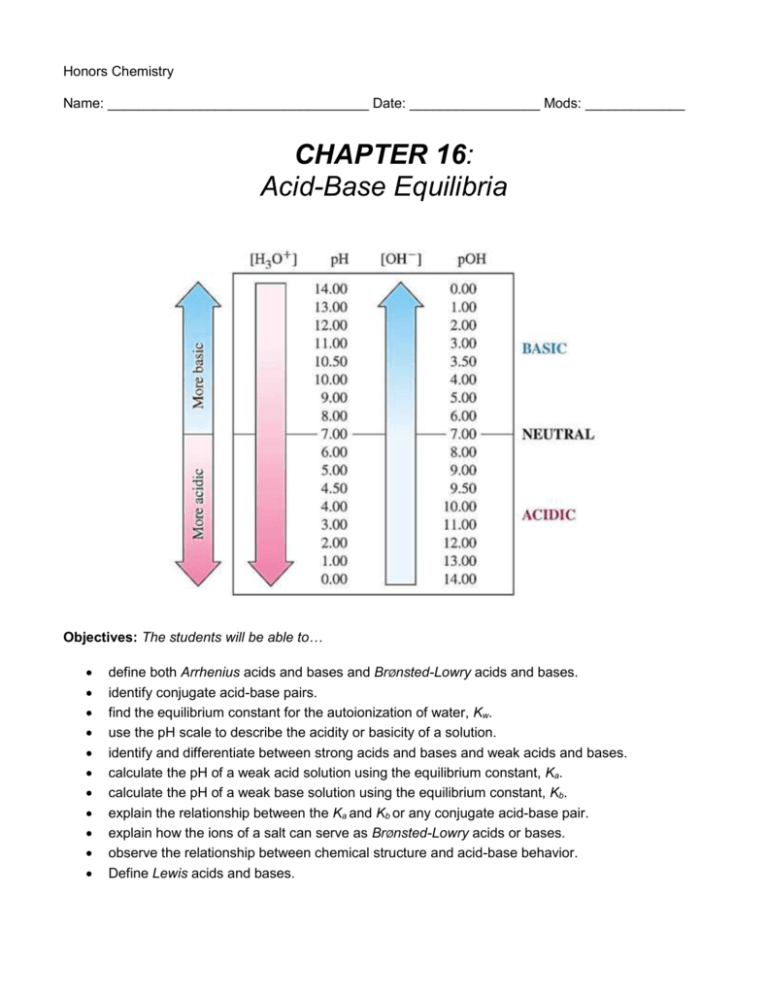
Honors Chemistry Name: __________________________________ Date: _________________ Mods: _____________ CHAPTER 16: Acid-Base Equilibria Objectives: The students will be able to… define both Arrhenius acids and bases and BrØnsted-Lowry acids and bases. identify conjugate acid-base pairs. find the equilibrium constant for the autoionization of water, Kw. use the pH scale to describe the acidity or basicity of a solution. identify and differentiate between strong acids and bases and weak acids and bases. calculate the pH of a weak acid solution using the equilibrium constant, Ka. calculate the pH of a weak base solution using the equilibrium constant, Kb. explain the relationship between the Ka and Kb or any conjugate acid-base pair. explain how the ions of a salt can serve as BrØnsted-Lowry acids or bases. observe the relationship between chemical structure and acid-base behavior. Define Lewis acids and bases. Chapter 16 Reading Outline: 16.1 – Acids and Bases: A Brief Review (pg. 670) 1. What are some basic properties of acids? Of bases? 2. State the Arrhenius concept of acids and bases. 16.2 – BrØnsted-Lowry Acids and Bases (pgs. 670-676) 1. Why is the Arrhenius concept of acids and bases limited? 2. An H+ ion is simply a _______________ with no surrounding ________________ electron. 3. Copy equation 16.2 below to show how a proton can interact with a water molecule to form the hydronium ion, H3O+ 4. Give the BrØnsted-Lowry definition of an acid and a base. 5. Explain the meaning of the word amphiprotic and provide an example of an amphiprotic substance. 6. Copy equation 16.7 below and use it to explain the term conjugate acid-base pair. 7. The ______________________ an acid, the _____________________ its conjugate base; the _______________________ a base, the _____________________ its conjugate acid. 8. Name and describe the three categories of acids and bases based on their behavior in water. 9. Describe the position of equilibrium in an acid-base reaction. 16.3 – The Autoionization of Water (pgs.676-678) 1. Copy equation 16.12 below to explain the autoionization of water. 2. If water can be autoionized, why is water a poor conductor of electricity? 3. Copy equation 16.16 below, which is extremely important and should be committed to memory. 4. When is a solution considered to be neutral? 5. When is a solution considered to be acidic? 6. When is a solution considered to be basic? 16.4 – The pH Scale (pgs. 678-682) 1. Use Table 16.1 to fill in the relationships among [H+], [OH-], and pH at 25°C below. Solution Type [H+] (M) [OH-] (M) pH Value 2. IMPORTANT: Because the pH scale is logarithmic, a change by 1 on the scale means a change in [H+] or [OH-] by a factor of _______. 3. What is the normal range for the pH of human blood? What happens if this range is not met? 4. We will use equations 16.17, 16.18 and 16.20 a great deal in class. Copy them below. 5. Name and describe some common tools used to measure pH. 16.5 – Strong Acids and Bases (pgs. 682-684) 1. Strong acids and strong bases are strong _________________________, existing in aqueous solutions entirely as ______________. 2. Find Table 4.2 on pg. 132 in Chapter 4. List the strong acids and strong bases below. 16.6 and 16.7 – Weak Acids and Weak Bases (pgs. 684-696) 1. Weak acids are only partially ionized in aqueous solutions. Copy equation 16.27 below to show how we can use the acid-dissociation constant (Ka) to express the extent to which a weak acid ionizes. 2. The larger the value of Ka, the _________________ the acid. 3. Read through the sections and example problems on pages 685-691 to familiarize yourself with how we can calculate Ka from pH and how we can calculate pH from Ka. 4. Define polyprotic acid and give an example. 5. It is always easier to remove the ______________ proton from a polyprotic acid than to remove the _______________. Thus, the Ka values become successively ______________ as successive protons are removed. 6. The constant Kb always refers to: 16.8 – Relationship Between Ka and Kb (pgs. 696-699) 1. Use Equation 16.40 and the paragraph it is embedded within to explain the relationship between Ka and Kb. 16.9 – Acid-Base Properties of Salt Solutions (pgs. 699-702) 1. Explain the process of hydrolysis (glossary definition may be easier to understand). 2. List the six rules for determining the effect of cations and anions in solution. 16.11 – Lewis Acids and Bases (pgs. 706-710) 3. Give the Lewis definition of an acid and a base. 4. Draw one example using Lewis structures to show the reaction between a Lewis acid and a Lewis base.








