Hillerson Natalie Hillerson TA: Jeremy Lehner 21 January 2014
advertisement

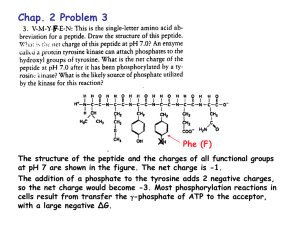
Hillerson 1 Natalie Hillerson TA: Jeremy Lehner 21 January 2014 Buffers I. Introduction The overarching purpose of this lab was to determine the buffering capacity of solutions containing different phosphate species, specifically HPO42- and H2PO4-, and to compare solutions prepared by dissolving aspirin and bufferin in water. The main concept in the lab was the buffer: a solution containing a weak acid (or a weak base) and its conjugate base (or conjugate acid). A good buffer will resist changes in pH upon the addition of a strong acid or base. The quality of a buffer is determined by its buffering capacity, which is defined quantitatively through the following equation: ∆[H + ] 𝛽= ∆pH In this equation, ∆[H+] is the concentration of H+ ions added to the buffer solution, and ∆pH is the subsequent change in pH. A good buffer will have a buffer capacity, as defined by this equation, which is fairly large (less than 1, but high compared to other, less effective buffers), meaning that the concentration of added hydrogen ions is smaller than but close to the change in pH, indicating that the buffer successfully resisted pH changes while protons were being added. A similar equation can be used for the addition of a strong base simply by replacing ∆[H+] with ∆[OH-]. These equations were used for both the phosphate species and the aspirin and bufferin comparisons. Hillerson 2 Buffers can be made with weak polyprotic acids, as was the case in this lab. Polyprotic acids are amphoteric, meaning the ions of a polyprotic acid can behave as both an acid and a base (accepting as well as donating protons) in successive deprotonations. The polyprotic acid used in this lab was H2PO4- and HPO42-, both of which are represented by the following chemical reactions: HPO42- + H2O ⇋ OH- + H2PO4- Kb = 1.61 x 10-7 H2PO4- + H2O ⇋ H+ + HPO42- Ka = 6.23 x 10-8 For the first equation listed, HPO42- acts as a base because it will dissolve partially in water to produce hydroxide ions and H2PO4-. This is why the Kb value was used as opposed to the Ka value, and this Kb value was found by dividing Kw (1.0 x 10-14) by the listed Ka value of the H2PO4- / HPO42- weak acid/conjugate base pair. II. Procedure First, 100 mL solutions of both 0.1 M H2PO4- and 0.1 M HPO42- were prepared by dissolving 1.3608 g of KH2PO4 and 1.742 g of K2HPO4 in 100 mL of water, respectively. Then, 40 mL of the HPO42- was transferred into a separate beaker and 60 mL of DI water was added, making solution 1. At this point, the pH probe was calibrated to samples of 4.00 pH and 10.00 pH. The pH of solution 1 was then measured and recorded. Next, 20 mL of this solution was again transferred into a separate beaker and .25 mL of .02 M HCl was added, the pH then recorded. After disposing of this solution in the waste container, another 20 mL sample of solution 1 was taken, but this time .25 mL of .1 M NaOH was added, the pH again recorded. This 20 mL solution, as well as solution 1 was then discarded in the appropriate Hillerson 3 container. This same procedure was followed for solutions 2-5, according to the Figure 1 in the Results section. For the second part of the lab involving aspirin and bufferin, 0.2 g of both substances were weighed and transferred to their own 125 mL beaker. The active ingredients of each substance were recorded. Then, 25 mL of DI water was added to each beaker, and the resulting solution was stirred. The solubility of each substance was also noted. After stirring, the pH of the two solutions was recorded. To each solution, 0.25 mL of 0.5 M HCl was added and the pH recorded. This entire process was then repeated, only adding 0.25 mL of 0.5 NaOH instead of HCl. III. Discussion The phosphate-containing solution that had the largest buffer capacity overall was solution 3, as shown in Figure 2, with the same mole amounts of H2PO4- and HPO42-. Because this solution contained equal amounts of an acid and its conjugate base to begin with, it more readily and easily reacted with the added protons and hydroxide ions and essentially “neutralized” this addition to a good degree. As seen in the lab, solution 3 did have the highest buffering capacity of any of the other solutions, with the exception of solution 4—the addition of OH- resulted in a calculated high buffer capacity. This may be due to human error in calculating the exact molarity of OH- ions to be added and actually adding a different, lower concentration, which resulted in the skewed calculation of buffer capacity. The solution with the lowest buffer capacity was solution 1. In this solution, only HPO42ions are present (dissociation of this substance into H2PO4- is assumed to be negligible due to Hillerson 4 the fact that HPO42- is a very weak base). Since only a weak base exists, and not its conjugate acid, the addition of H+ or OH- will change the pH more dramatically compared to solution 3, the better buffer. Solution 5 is also presumed to be an ineffective buffer, but to a somewhat lesser extent than solution 4. Although solution 5 was not tested in lab, subsequent predictions and calculations indicate that it would also be a poor buffer for the same reasons listed for solution 1—there is only H2PO4- present in solution. Both the aspirin and bufferin solutions had very high buffering capacities—higher than any of the phosphate-containing species. Aspirin was a better buffer than bufferin, if only slightly. This is likely because even though the gram amount of both substances was the same (0.2 g), the chemical makeup of these two compounds is different, and thus there were different mole amounts in solution. Bufferin has additional inactive ingredients than aspirin, so aspirin was shown to be the better buffer perhaps because the dissolved aspirin was more concentrated in acid/conjugate base pairing, whereas the dissolved bufferin solution had added inert species present in solution, making the acid/conjugate base pairing more dilute and less effective as a buffer. Additionally, it is difficult to compute a theoretical buffering capacity value for aspirin and bufferin, because both substances have complex chemical makeups with many components that may affect their buffering capacities in ways that are unknown and cannot be mathematically evaluated. Still, theoretically and experimentally, aspirin has the higher buffer capacity than bufferin. The experimentally calculated values of buffering capacity for the addition of H+ ions to the phosphate-containing solutions compare quite well to the theoretical values of β, as seen in Figure 3. For the majority of the solutions, the magnitudes of the values were the same, and the numerical values were quite close as well. The only discrepancy evident was solution Hillerson 5 5. The theoretical value was quite small compared to the experimental value, and this might be because the experimental value for solution 5 was an approximation, as this data was not collected in the lab. According to Figure 4, the pH range in which the buffering capacity is highest is around 7 and a bit higher, perhaps ranging from 7-8. This range of pH makes sense, as the pKa of the H2PO4- / HPO42- weak acid/conjugate base pair is 7.21, and according to the HendersonHasselbalch equation (pH = pKa + log([A-]/[HA]), the pH equals the pKa when the ratio of acid to conjugate base is equal to 1. A highly effective buffer forms when these two concentrations are equal to 1, and as such, the pH of this buffer in question will be 7.21. For the first deprotonation of H3PO4, the acid/conjugate base pairing is H3PO4 / H2PO4-, with the pKa value being 2.12. Therefore, the most effective pH for a buffer containing H3PO4 and H2PO4- would be 2.12. Likewise, for the pairing of HPO42- and PO43-, which has a pKa of 12.32, the most effective pH for this buffer would be 12.32. IV. Conclusion This lab dealt entirely with buffers and their respective buffer capacities. This lab demonstrated that the best buffers result from equal amounts of acid and conjugate base present in solution before the addition of a strong acid or base (solution 3 is an accurate demonstration of this statement). Likewise, those solutions with uneven concentrations of acid and conjugate base at the start proved to be poor buffers with small buffer capacities. Similarly, aspirin and bufferin both made highly effective buffers. Aspirin had a higher buffer capacity than bufferin because its chemical makeup was less complex and therefore Hillerson 6 had more dissolved acid/conjugate base than bufferin, which had more dissolved species not associated with buffers. V. Calculations - Calculation of experimental buffer capacity: Solution 1: ∆[H+] = M1V1 = M2V2 = (.02)(.00025) = (x)(.02025) ∆pH = |initial pH – pH after addition of acid| = |9.06-8.83| = .23 ∆[H+] / ∆pH = experimental buffer capacity, β = 1.07 x 10-3 Similar calculations were done for [OH-] and for solutions 2-5, DI water, aspirin, and bufferin. - Calculation of theoretical buffer capacity: β = ln(10) x ((Ka x [H+]) / (Ka + [H+])^2)) x C, where C = [HA] + [A-] Solution 1: Ka = 6.23 x 10-8, [H+] = 10-8.83 (8.83 is the pH after addition of HCl), C = .04 M (dissociation is assumed to be negligible). Using these values, β = 1.43 x 10-3 Similar calculations were done for solutions 2-5.