detecting signs of chemical change
advertisement

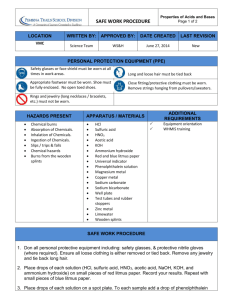
DETECTING SIGNS OF CHEMICAL CHANGE EXPERIMENT: 399 DATE: HEATHER FRENCH SECTION 62 Abstract: Detecting signs of chemical change were conducted in this experiment through the mixing of liquids, solutions and solids, while observing their characteristic appearance. Laboratory observations were determined and recorded if mixing each combination resulted in a chemical change. 12 different liquids, solutions and solids were reacted in 12 different test tubes to see if a chemical or physical change occurred. It was concluded that all assignments with the exception of E – copper(II) sulfate and hydrochloric acid; CuSO4 + 2HCl → CuCl2 + H2 SO4 – produced a chemical change of either a precipitate, evolution of a gas, a color change or temperature change. The copper(II) sulfate and hydrochloric acid solution did not form a precipitate because copper(II) chloride is soluble in aqueous solution and will dissociate. This experiment successfully proved that a significant amount of substance, when mixed together, will result in a chemical change. Introduction: The purpose of this lab was to observe specific experimental reactions and identify if a physical or chemical change occurred. Typical indications of a physical change include a phase change, such as when liquid water freezes or boils and the changing of the reactions appearance without chemical compositional change. Typical chemical change characteristics include the formation of a precipitate, color change that does not simply represent the dilution of either solutions, evolution of a gas and a temperature change. Every chemical reaction involves a transfer of energy. In many cases, this energy transfer involves easily detectable heat or thermal energy changes. A heat change may cause an increase in temperature, exothermic, or a decrease in temperature, endothermic. During this lab it was critical to use sight, touch and smell to detect the physical or chemical changes. Materials and Equipment: Chemicals: 0.1 M cobalt(II) chloride hexahydrate 95% ethanol 0.1 M cobalt(II) chloride hexahydrate dissolved in 95% ethanol water zinc 1.0 M hydrochloric acid solution 0.1 M copper(II) sulfate solution 0.5 M sodium hydrogen carbonate solution 1.0 M ammonium solution magnesium 1.0 M sodium hydroxide solution ammonium chloride Distilled water Materials/Equipment: Test tubes (12) Absorbent paper towel Wax weighing paper Microspatula Pipets 10 mL graduated cylinder Sandpaper Waste beaker Procedure: 12 test tubes were washed with soap, thoroughly rinsed, flushed with 5 mL of deionized water and placed on a rack to dry. The 12 test tubes represented one clean, dry test tube for each of the 12 assignments. First, 2 mL of 95% ethanol was added to a clean dry test tube containing a small amount of CoCl2 • 6 H2O and mixed well. Observations were recorded and the contents of the test tube were properly discarded. Next, 1 mL of distilled water was added to a clean test tube containing 1 mL of CoCl2 • 6 H2O in 95% ethanol solution and mixed well. Observations were recorded and the contents of the test tube were properly discarded. Then, 2 mL of 1.0 M HCl was added to a clean test tube containing a piece of Zn about half the size of a pea. The solution was mixed well and observations were recorded on the Data Sheets. Afterwards contents of test tube were properly disposed of. Next, 2 mL of a 0.1 M CuSO4 solution was added to a clean, properly labeled test tube containing a piece of Zn about half the size of a pea. The solution was then mixed well and the reaction was observed for specific signs of chemical changes. All data was recorded in the Data Sheet and contents of test tube were properly discarded. A new, clean, dry test tube was obtained from the drying rack and labeled appropriately. Next, 1 mL of 0.1 M CuSO4 was transferred to the test tube, to which 1 mL of 1.0 M HCl solution was added and mixed well. Then all observations were recorded in the Data Sheet and the waste was disposed of properly. Another clean, dry test tube was obtained from the drying rack and labeled appropriately. Next, 1 mL 0.5 NaHCO3 was added to the test tube containing 1 mL of 0.1 M CuSO4. The solution was then mixed well and all observations were recorded on the Data Sheet. Assignment letter G was conducted by adding 1 mL of 1 M NH3 solution to a clean, dry test tube containing 1 mL of 0.1 M CuSO4. The solution was mixed thoroughly and all observations were recorded on the Data Sheet. After, all waste was disposed of properly. Next, a 1 cm strip of Mg was rubbed with sandpaper to remove the oxide coating and give it a shinny appearance. The Mg was transferred to a clean, dry test tube to which 2mL of 0.1 M CuSO4 was added and mixed thoroughly. Then all reaction observations were recorded on the Data Sheet. Another shiny 1 cm strip of Mg was added to a new, clean, dry test tube. 2 mL of 1.0 M HCl solution was added to the test tube containing the strip of Mg and all reaction observations were recorded on the Data Sheet. Contents of test tube were then discarded properly. Next, 1 mL of 1.0 M NaOH solution was added to a clean, dry test tube containing 1 mL of 1.0 M HCl. The reaction observations were then recorded on the Data Sheet and the waste of assignment J was disposed of properly. Assignment K was conducted by adding 1 mL of 0.5 M NaHCO3 to a clean, dry test tube containing 1 mL of 1.0 M HCl and mixing thoroughly. The reaction observations were then recorded on the Data Sheet and the waste was disposed of properly. Lastly, Experiment 399 – Detecting signs of Chemical Change – was concluded with the reaction of NH4Cl and H2O. A small amount of NH4Cl solid was transferred to a clean, dry test tube. Then 2 mL of room temperature distilled water was added to the test tube containing the NH4Cl solid and mixed thoroughly. All reaction observations were recorded on the Data Sheet and the waste contents of assignment L were disposed of properly. Results: Observations – Over the course of this experiment a number of observations were made for each of the twelve reactions. One that is more interesting than the others is assignment E. This was the only reaction in which a chemical change did not occur. In fact the solution was only slightly less blue, no visible reaction took place. Assignment A and B were two reactions that resulted in a color change. A changed from pink to blue and B changed from blue to pink. Each contained a distinct smell of alcohol due to the presence of ethanol (grain alcohol). Assignment C and I produced similar results. Each reaction completely dissolved the solid and produced a vigorous hydrogen gas that bubbled off the Zn and Mg. The reaction involving Mg and HCl was significantly more reactive because of the heat and condensation produced, resulting in an exothermic reaction. Data/Calculations – See original data sheets attached. Discussion/Conclusion: This experiment solidified the prediction that a significant number of reactions can be classified as chemical changes. The results indicated that all the assignments were chemical changes except for assignment E. All the assignment reactions produced various characteristics of a chemical change, from gaseous bubbles to a precipitate and from color change to heat exchange. The data yielded only one reaction that produced a precipitate, indicating that the other products were soluble in aqueous solutions or that they produced only a physical change. It was concluded in this experiment that mixing solutions, not solids, together formed a precipitate and that the changing of the solutions color did not necessarily mean that a precipitate had formed as in assignment A,B,C,D,G,H and I. One reaction that was significantly interesting was assignment L - NH4Cl + H2O. This reaction was observed to decrease in temperature, resulting in an endothermic reaction.