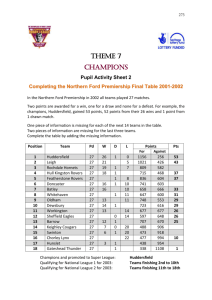
Chemistry - Huddersfield New College
advertisement

Pre-course task for 2015 entry. AS Chemistry Why do I need to complete a pre-course task? The task serves two purposes. Firstly, it allows you to carry out a little bit of preparation before starting your studies in September. Secondly, the task is designed to allow the college to assess important skills at the start of the course, which they can use for planning student activities, identifying where support might be needed and to help in the assessment of your suitability for the AS level course. We have found that some students elect to take an AS level without recognising the skills needed to receive a high grade in an examined course; such students may be better suited to a vocational qualification, which is only assessed by coursework. Your performance in the task will allow your teachers to support you fully to ensure you are on the right course to maximise your performance. When should I hand it in? You should have completed this task by the first lesson in September. You should hand it in to your subject teacher on that day. How will I be given feedback on how well I have done? Feedback will be given in written and verbal form and will outline your strengths and areas for development. This work comprises an element of the assessment used during induction. Task In Detail You should complete questions 1-12 fully. A periodic table and a table of common ions has been provided. Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk Course: AS Chemistry Student name: Date submitted: 1. Complete the following table. The first few have been done for you element symbol atomic number hydrogen H 1 helium He lithium Li mass number 1 number of protons 1 number of neutrons 0 number electron of arrangement electrons 1 1 2 4 2 2 2 2 3 7 3 4 3 2,1 beryllium 4 9 boron 5 11 carbon 6 12 nitrogen 7 14 oxygen 8 16 fluorine 9 19 neon 10 20 sodium 11 23 magnesium 12 24 aluminium 13 27 silicon 14 28 phosphorus 15 31 sulphur 16 32 chlorine 17 35 argon 18 40 potassium 19 39 calcium 20 40 Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk 2. Neatly draw diagrams to show the electron arrangement of an atom for the following elements. a. nitrogen b. carbon c. potassium d. phosphorus e. argon Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk 3. Ionic bonding. Draw a dot and cross diagram that shows the ionic bonding in the following compounds. Lithium Fluoride (LiF) Calcium Fluoride (CaF2) Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk Aluminium Oxide (Al2O3) 4. Using the Periodic Table, write the symbol for the ion formed (including its charge) from each of the following elements: element symbol of ion formed element lithium fluorine rubidium iodine calcium astatine strontium selenium gallium phosphorus symbol of ion formed 5. Fill in the table to show the electron arrangements for the following ions: ion electron arrangement ion K+ F- S2- Mg2+ Al3+ N3- Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk electron arrangement 6. Formulae: Using the table of common ions provided complete the following table by writing in the symbols for all the ions, positive (cations) and negative (anions), which are part of the named compounds. Hence work out the formulae of the compounds. You are permitted to use a Periodic Table. Name of Compound Symbol for Symbol for Formula of positive ion negative ion Compound (cation) (anion) potassium iodide rubidium oxide sodium nitride beryllium fluoride magnesium oxide aluminium oxide sodium nitrate magnesium hydroxide magnesium nitrate calcium phosphate ammonium sulphate copper(II) carbonate iron(II) sulphate sodium hydrogencarbonate Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk Covalent bonding and properties of covalent compounds 7. Draw ‘dot and cross’ diagrams showing all the electrons in the following molecules: Fluorine (F2) Hydrogen sulphide (H2S) Carbon dioxide (CO2) Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk Phosphine (PH3) Nitrogen (N2) 8. Balancing Equations. Balance the following equations: C2H6(g) + O2 (g) → Fe(s) + Na(s) + KI(aq) + CaCO3(s) + O2(g) → H2O(l) → → → PbI2(s) + CaCl2(aq) Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk H2O(l) Fe2O3(s) NaOH(aq) + Pb(NO3)2(aq) HCl(aq) CO2 (g) + + H2(g) KNO3(aq) CO2(g) + H2O(l) Question 9, 10, and 11 involve chemical calculations 9. Rubidium forms an ionic compound with silver and iodine. This compound has a potential use in miniaturised batteries because of its high electrical conductivity. The empirical formula of this ionic compound can be calculated from its percentage composition by mass: Rb, 7.42%; Ag, 37.48%; I, 55.10%. (i) Define the term empirical formula. ......................................................................................................................... ......................................................................................................................... ......................................................................................................................... (ii) Calculate the empirical formula of the compound showing your working clearly. Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk 10. Barium metal can be extracted from barium oxide, BaO, by reduction with aluminium. 6BaO + 2Al 3Ba + Ba3Al2O6 Calculate the mass of barium metal that could be produced from reduction of 500 g of barium oxide using this method. Answer = ............................... g 11. (a) Balance the following equation. CaCO3 + HNO3 Ca(NO3)2 + CO2 + H2O (b) What mass of calcium nitrate would be obtained from 40grams of calcium carbonate in the above reaction. Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk 12. Giant Covalent Structures formed by carbon atoms. (Fill in the blanks and answer the questions.) Diamond In the structure each carbon is joined to _________ other carbon atoms by ___________________ bonds consisting of _____________________ electrons. The arrangement around each carbon is __________________________and the angles are all _______ degrees. http://www.hull.ac.uk/chemistry/intro_inorganic/Diamond.htm Explain why diamond has a high melting temperature Explain why diamond does not conduct electricity Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk C-C-C bond Graphite http://facweb.bhc.edu/academics/science/harwoodr/GEOL101/study/minerals.htm Each carbon atom is bonded to __________________ other carbon atoms by _______________ bonds, forming hexagonal rings. The fourth outer electron of each carbon atom is delocalised between the sheets of hexagons. The interactions between the layers of hexagons are _________________ so the layers can slip over each other. Explain why graphite has a high melting temperature Explain why graphite conducts electricity Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk Table of common ions +1 +2 +3 -3 -2 -1 lithium Li+ beryllium Be2+ aluminium Al3+ nitride N3- oxide O2- fluoride F- sodium Na+ magnesium Mg2+ iron(III) Fe3+ phosphide P3- sulphide S2- chloride Cl- potassium K+ calcium Ca2+ phosphate PO43- carbonate CO32- bromide Br- rubidium Rb+ strontium Sr2+ sulphate SO42- iodide I- caesium Cs+ barium Ba2+ hydroxide OH- silver Ag+ iron(II) Fe2+ nitrate NO3- copper(I) Cu+ zinc Zn2+ hydrogencarbonate HCO3- hydrogen H+ Copper(II) Cu2+ hydrogensulphate ammonium NH4+ Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk HSO4- Huddersfield New College, New Hey Road, Huddersfield, HD3 4GL Telephone: 01484 652341 email: info@huddnewcoll.ac.uk www.huddnewcoll.ac.uk