Report Form - The Health Products Regulatory Authority
advertisement
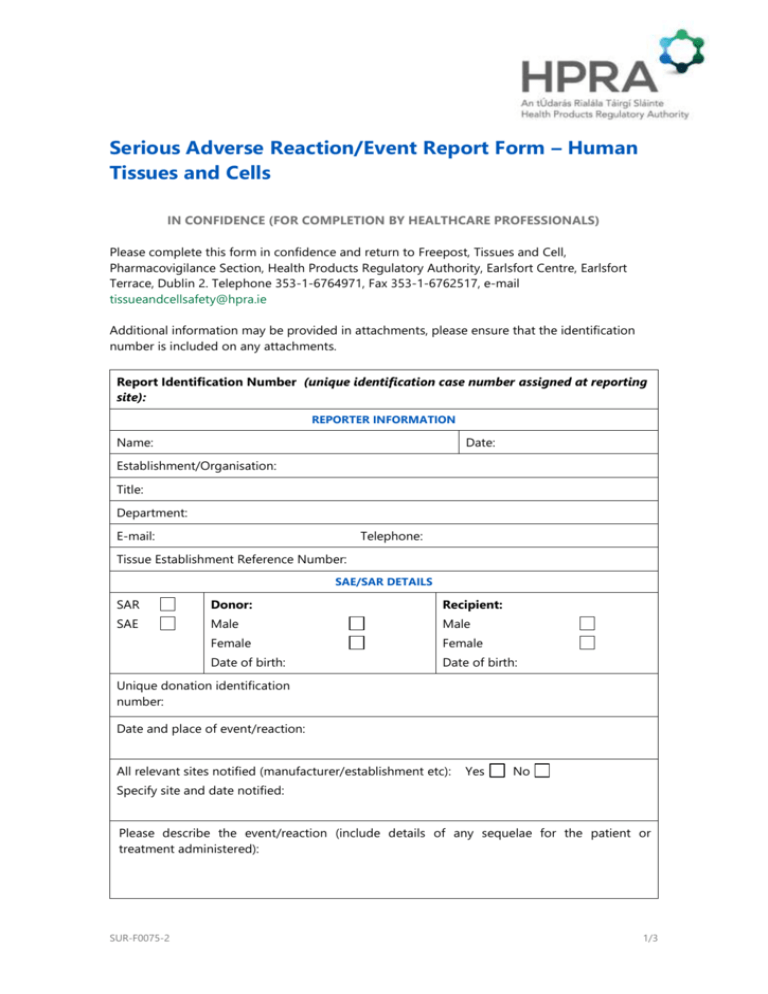
Serious Adverse Reaction/Event Report Form – Human Tissues and Cells IN CONFIDENCE (FOR COMPLETION BY HEALTHCARE PROFESSIONALS) Please complete this form in confidence and return to Freepost, Tissues and Cell, Pharmacovigilance Section, Health Products Regulatory Authority, Earlsfort Centre, Earlsfort Terrace, Dublin 2. Telephone 353-1-6764971, Fax 353-1-6762517, e-mail tissueandcellsafety@hpra.ie Additional information may be provided in attachments, please ensure that the identification number is included on any attachments. Report Identification Number (unique identification case number assigned at reporting site): REPORTER INFORMATION Name: Date: Establishment/Organisation: Title: Department: E-mail: Telephone: Tissue Establishment Reference Number: SAE/SAR DETAILS SAR Donor: Recipient: SAE Male Male Female Female Date of birth: Date of birth: Unique donation identification number: Date and place of event/reaction: All relevant sites notified (manufacturer/establishment etc): Yes No Specify site and date notified: Please describe the event/reaction (include details of any sequelae for the patient or treatment administered): SUR-F0075-2 1/3 IMPLICATED TISSUES/CELLS (SELECT ALL THAT APPLY) Non ART Autologous Allogeneic Heart valves Other cardiovascular, please specify Vessels Bone Tendons Demineralised bone Ligaments Other musculoskeletal, please specify Cornea Sclera Bone marrow Peripheral blood stem cells Donor lymphocyte infusions Skin Other ocular, please specify Umbilical cord blood Other stem cells, please specify Hepatocytes Amniotic membrane Pancreatic islets Others, please specify ART Partner Sperm Non-partner Oocytes Embryo Ovarian tissue OTHER DETAILS Date and place of procurement: Date and place of human application: SAR CATEGORISATION Transmitted bacterial infection Transmitted viral infection Transmitted parasitical infection Transmitted malignant disease Other disease transmissions Other, please specify SAE CATEGORISATION Did the event occur at: Procurement Testing Transport Distribution Materials Other, please specify SUR-F0075-2 Processing Storage 2/3 Specification: Tissue and cells defect Equipment failure Human Error Other, please specify ROOT CAUSE ANALYSIS SAR/E Please provide details: CORRECTIVE AND PREVENTATIVE ACTIONS SAR/E Please provide details: FOR SARS ONLY PROVIDE CLINICAL OUTCOME (IF KNOWN): Complete recovery Signature: Minor sequelae _______________________________________ Serious sequelae Death Date: ________________________ Thank you for taking the time to complete this form. Please note that by your completion of this report form, we understand that you are consenting to the information provided, including your contact details, to be stored securely by the HPRA. Your contact details will be used solely for the purposes of interaction with you regarding this report. For the purposes of complying with our statutory and legal reporting requirements, summary details of this report (excluding personal information) may be shared with other bodies also involved in safety monitoring in accordance with data protection requirements. The right exists to request a copy of personal data held by the HPRA and to have any inaccuracies in such data corrected or deleted. SUR-F0075-2 3/3