Tween-20/ H 2 O Promoted Green Synthesis, Computational and
advertisement

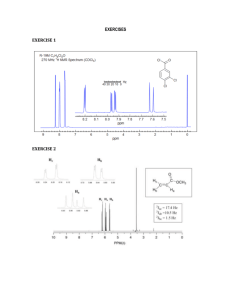
S1 Tween-20/ H2O Promoted Green Synthesis, Computational and Antibacterial Activity of Amino Acid Substituted Methylene Bisphosphonates S. Siva Prasada, S.H. Jayaprakasha, Ch. Syama sundara, P. Sreelakshmia, C. Bhuvaneswarb,c, B. Vijaya Bhaskarb, W. Rajendrab and C. Suresh Reddya,* a b Department of Chemistry, Sri Venkateswara University, Tirupati - 517 502, INDIA Division of Molecular Biology, Department of Zoology, Sri Venkateswara University, Tirupati – 517 502, INDIA c Department of Microbiology, Sri Venkateswara University, Tirupati - 517 502, INDIA E-mail: csrsvu@gmail.com Supplemental Materials Biological Activity Ciprofloxacin was used as standard antibacterial agent. Solutions of the test compounds and reference drug were prepared in dimethyl sulfoxide (DMSO) at concentrations of 100, 50, 25, 12.5, 6.25, 3.125 and 1.5625 µg/ mL. Nine tubes were prepared in duplicate with the second set being used as MIC reference controls (16-24 h visual). After sample preparation, the controls were placed in a 37°C incubator and read for macroscopic growth (clear or turbid) the next day. Into each tube, 0.8 mL of nutrient broth was pipetted (tubes 2–8), tube 1 (negative control) received 1.0 mL of nutrient broth and tube 9 (positive control) received 0.9 mL of nutrient. Tube 1, the negative control, did not contain bacteria or antibiotic. The positive control, tube 9, received 0.9 mL of nutrient broth since it contained bacteria but not antibiotic. The test compound were dissolved in DMSO (100 µg/ mL), 0.1 mL of increasing concentration of the prepared test compounds which are serially diluted from tube 2 to tube 8 from highest (100 µg/ mL) to lowest (1.5625 µg/ mL) concentration (tube 2-8 containing 100, 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL). S2 After this process, each tube was inoculated with 0.1 mL of the bacterial suspension, concentration of which corresponded to 0.5 McFarland scale (9 X 108 cells/ mL) and each bacterium was incubated at 37 °C for 24 h. The final volume in each tube was 1.0 mL. The incubation chamber was kept humid. At the end of the incubation period, MIC values were recorded as the lowest concentration of the substance that gave no visible turbidity, i.e. no growth of inoculated bacteria. The results were shown in Table S 1. Table S 1: Results of antibacterial activity of 4a-j: MIC values in μg/ mL. Gram -ve Gram +ve Compounds Klebsiella pneumonia Pseudomonas aeruginosa Staphylococcus aureus Bacillus subtilis 4a 1.5625 3.125 3.125 1.5625 4b >100 >100 50 25 4c 50 >100 >100 50 4d 1.5625 1.5625 1.5625 1.5625 4e 50 >100 >100 >100 4f 50 50 3.125 3.125 4g >100 >100 25 25 4h 3.125 50 >100 >100 4i 50 50 25 50 4j 50 25 >100 >100 Ciprofloxacin 1 2 2 2 S3 Table S 2: Antibacterial activity of Compounds4a-j: Zone of Inhibition (mm). Zone of Inhibition (mm) Compound Gram -ve Klebsiella Pseudomonas pneumonia aeruginosa 50 100 50 100 µg/mL µg/mL µg/mL µg/mL Gram +ve Staphylococcus Bacillus subtilis aureus 50 100 50 100 µg/mL µg/mL µg/mL µg/mL 4a 33 36 18 25 25 30 21 28 4b 25 32 16 18 18 20 8 11 4c 26 32 14 16 20 29 10 10 4d 30 34 21 28 30 33 27 31 4e 26 31 17 17 0 21 2 4 4f 25 32 12 19 15 20 10 21 4g 26 29 11 20 22 24 4 12 4h 4i 25 28 30 31 14 10 19 18 25 17 26 28 18 12 20 24 4j 25 30 13 16 0 25 1 7 Gentamycin 18 25 33 24 S4 Table S 3: Hydrogen bonding interactions between protein and ligands 4a-j Compound 4a 4b 4c 4d 4e 4f 4g Interactions Protein ligand Asp517NH1------------OS13 Asp517NH2------------ OS12 Asp517NH2------------ OS13 Arg1012NH2-----------OC24 Ser1021OG-------------OC6 Ser1021OG-------------OP12 Ser1028OG-------------OP15 Arg1033NH2-----------OP13 Arg1048NH1-----------OC24 Arg1048NH2----------- OC24 His1081NE2------------OC25 Asp510CO-----------HO21 Asp510CN----------HO21 Arg517NH2--------OC21 Arg517NH2--------OP24 Ser1021OG---------OP22 Asp1024OD2-------OD2 Asp508OD2-----------HN24 Lys581NZ------------OP21 Lys581NZ------------OP23 Lys581NZ------------OP19 Gly584N--------------OC32 Gln1095NE2---------OC34 Ser1098OG--------------OP24 Ser1112N-------------OC35 Arg1092NH1-----------OP10 Gln1095OE1------------HN14 Ser1098OG-------------OP10 Ser1098OG-------------OP11 Ser1112CO--------------HO2 Asp1116CO------------- HO2 Thr507CO---------------HO3 ARg517NH1-------------OC31 Distance (Å) 3.3 2.7 2.8 3.2 3.0 3.2 3.2 3.0 3.0 3.2 3.2 2.5 3.2 3.1 3.3 2.8 2.2 2.6 3.1 3.0 3.1 2.9 2.8 3.1 3.3 3.2 2.4 3.2 2.8 2.6 2.4 2.0 3.2 Binding energy (Kcal/ mol) -6.8 -6.5 -5.8 -6.8 -7.1 -5.8 -6.1 S5 Compound 4h 4i 4j Ciprofloxacin Interactions Protein ligand Arg517NH2--------------OP12 Arg517NH2------------- OP13 Glu541NE2--------------OP10 Ser1021OG--------------OP12 Asp508CO---------------OC9 ARg517NH1-------------OC31 Arg517NH2--------------OC27 Arg517NH2------------- OP15 Arg517NH2------------- OP17 Glu541NE2--------------OP20 Thr544Og1--------------OP16 Arg1092NH1-----------OP15 Gln1095OE1-----------ON22 Gln1095NE1-----------OC27 Ser1112N---------------OC27 Asp1116CO-------------OC7 Val1268CO--------------HN5 Arg517NH2-------------OC31 Asp510N-----------------O31 Asp510O-----------------HO31 Ser1021OG--------------OP19 Ser1021OG------------OP23 Asp1024OD2------------HN24 Tyr580CO---------HN25 Ser1028OG-------OC23 Arg1033NH2------OC23 His1081NE2-------OC22 Distance (Å) 3.0 3.2 2.9 3.1 2.8 2.9 2.8 3.0 3.4 3.3 3.1 3.3 2.7 3.3 3.3 2.5 2.3 3.1 3.2 2.1 3.1 3.1 2.2 2.2 2.7 3.0 3.3 Binding energy (Kcal/ mol) -6.6 -6.2 -7.4 -6.8 S6 4j 4d 4h 4a Ciprofloxacin Figure S 1: Binding behavior of Compounds 4j, 4d, 4h, 4a and Ciprofloxacin with residues of metal binding actives sites of Type IIA topoisomerase S7 Figure S 2: 1H NMR Spectrum of compound 4a S8 Figure S 3: 13C NMR Spectrum of compound 4a S9 Figure S 4: 31P NMR Spectrum of compound 4a S 10 Figure S 5: 1H NMR Spectrum of compound 4d S 11 Figure S 6: 13C NMR Spectrum of compound 4d S 12 Figure S 7: 31P NMR Spectrum of compound 4d