Sig Figs: Activity 1.3 Group Members: Objective To understand how
advertisement
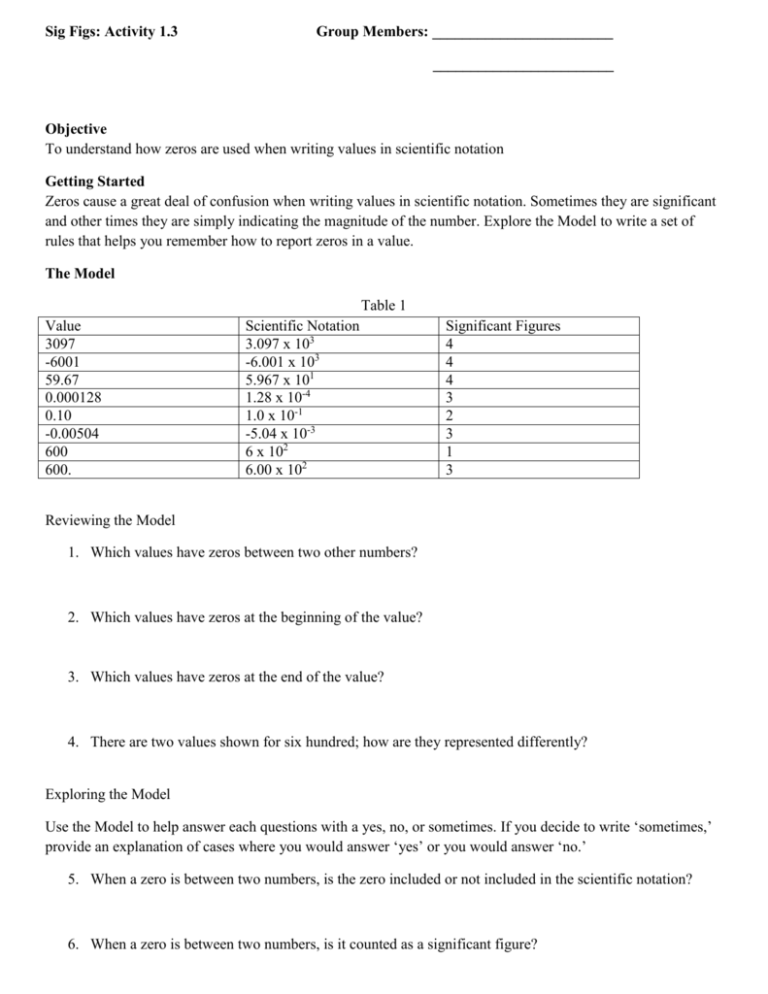
Sig Figs: Activity 1.3 Group Members: ________________________ ________________________ Objective To understand how zeros are used when writing values in scientific notation Getting Started Zeros cause a great deal of confusion when writing values in scientific notation. Sometimes they are significant and other times they are simply indicating the magnitude of the number. Explore the Model to write a set of rules that helps you remember how to report zeros in a value. The Model Table 1 Value 3097 -6001 59.67 0.000128 0.10 -0.00504 600 600. Scientific Notation 3.097 x 103 -6.001 x 103 5.967 x 101 1.28 x 10-4 1.0 x 10-1 -5.04 x 10-3 6 x 102 6.00 x 102 Significant Figures 4 4 4 3 2 3 1 3 Reviewing the Model 1. Which values have zeros between two other numbers? 2. Which values have zeros at the beginning of the value? 3. Which values have zeros at the end of the value? 4. There are two values shown for six hundred; how are they represented differently? Exploring the Model Use the Model to help answer each questions with a yes, no, or sometimes. If you decide to write ‘sometimes,’ provide an explanation of cases where you would answer ‘yes’ or you would answer ‘no.’ 5. When a zero is between two numbers, is the zero included or not included in the scientific notation? 6. When a zero is between two numbers, is it counted as a significant figure? 7. When a zero is at the beginning of a value, is the zero included in the scientific notation? 8. When a zero is at the beginning of a value, is it counted as a significant figure? 9. When a zero is at the end of a value, is the zero included in scientific notation? 10. When a zero is at the end of a value, is the zero included in the scientific notation? 11. What is the relationship between the coefficient of a number in scientific notation and the number of significant figures? 12. Does it make a difference whether a value is positive or negative when counting the number of significant figures? Exercising Your Knowledge 13. Complete the following table: Value 2014 Table 2 Scientific Notation Significant Figures 4 -7.20 x 105 0.0094 5964.20 8.2 x 103 2.841 x 10-4 14. How can significant figures and scientific notation quickly help a reader assess how well we know a measured quantity? 15. From Activity 1.2, if Anni predicted there were 4.82 x 102 beans in a jar, and Bart predicted that there were 5 x 102 beans, explain which of the two reported values is more precise.