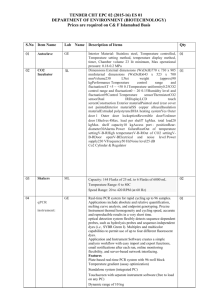
Legionella isolation scheme: pilot molecular method
advertisement

Store LENTICULE® samples at -20°C ± 5°C Food and Environmental Proficiency Testing Unit Laboratory identification no. (check): Please Enter your Lab No. Dispatch date: 2 November 2015 Final date for return of results: 4 December 2015 Results may be returned by fax, mail or email Contact details: The Organisers - FEPTU Public Health England 61 Colindale Avenue, London, NW9 5EQ, UK. Fax: +44 (0) 20 8200 8264 Tel: +44 (0) 20 8327 7119 e-mail: foodeqa@phe.gov.uk Legionella PCR Scheme PILOT DISTRIBUTION - Request/Report Form Distribution No: PILOT Download the safety data sheet: Sample numbers: GXA and GXB www.gov.uk/government/publications/safety-data-sheet-for-lenticules If you cannot examine any of these samples return your results as ‘Not examined’ Request: (i) (ii) Examine for the presence of legionellae Quantify legionellae in samples Results Isolation of legionellae (enter detected or not detected (ND)) Final Results Identification (enter L. pneumophila, species name or ‘not pneumophila’) Quantification GU L-1 FEPTU815.01 GXA GXB Detected Detected L.pneumophila detected Not pneumophila detected 8.2x104 1.5x102 Page 1 of 2 Laboratory identification no. (check): Please Enter your Lab No. We would be grateful if you would answer the following questions before returning this form to help us with the scheme development: Store LENTICULE® samples at -20°C ± 5°C 1. Which standard(s)/guidelines did you refer to for the examination these samples? ____ISO 12869_____________________________________________________________ 2. Does your laboratory routinely filter samples? If not, please provide details of your concentration method(s) ______________________________________________ 3. Please give details of primers (e.g. in house / commercial kit) _________________________________________________ 4. Describe your DNA extraction method (including volumes) ______________________________________________ 5. What volume of the extracted DNA used in PCR? _______________________________________ 6. Please provide details of master mix and volume used ___________________________________________ 7. What are your limit of detection and limit of quantification? ______________________________________________ 8. Does your laboratory routinely carry out both detection and quantification tests on samples? _____________________________________________________________ 9. Is your laboratory currently participating in any other legionella PCR scheme? _____________________________________________________________ 10. Please provide any further comment/s that will help FEPTU to ensure the scheme is appropriate when launched: __________________________________________________________________________ Authorised by: FEPTU815.01 Date reported: Page 2 of 2