The Nuclear Atom - Review Questions 23 marks
advertisement
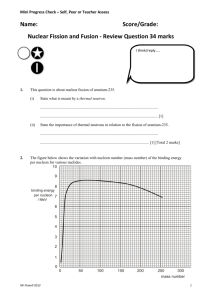
Mini Progress Check – Self, Peer or Teacher Assess Name: Score/Grade: The Nuclear Atom - Review Questions 23 marks I think/reply..... 1) Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes it suitable for this experiment. In your answer, you should make clear how evidence for the size of the nucleus follows from your description. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... [Total 8 marks] Mr Powell 2013 1 Mini Progress Check – Self, Peer or Teacher Assess 2) Explain what is meant by the statement that the strong interaction is a short-range force and explain what this implies about the densities of nuclei of various sizes. ................................................................................................................................. ................................................................................................................................. ................................................................................................................................. ................................................................................................................................. ................................................................................................................................. ................................................................................................................................. ................................................................................................................................. [Total 3 marks] 3) A uranium-236 nucleus, , and tellurium-131, (a) 236 92 U , 131 52 Te . undergoes fission, producing nuclei of zirconium-100, 100 40 Zr Write a nuclear equation to represent this fission reaction. ........................................................................................................................ [1] (b) Each of the product nuclei is a – emitter. (i) State the change, if any, in the nucleon number and the proton number caused by a – emission. nucleon number .................................................................................... proton number ...................................................................................... [1] (ii) The – decay of zirconium-100 is followed by three more – decays before the product nucleus is stable. State the nucleon number and the proton number of the resulting stable nucleus. nucleon number .................................................................................... proton number ...................................................................................... [1] Mr Powell 2013 2 Mini Progress Check – Self, Peer or Teacher Assess 3) Cont.. (iii) On the figure below, use crosses to represent each of the nuclei involved in the series of decays by which zirconium-100 changes to a stable nucleus. Add arrows to show the direction of each reaction. 62 60 58 neutron number 56 54 52 50 38 40 42 44 46 48 50 proton number [2] (iv) On a graph of neutron number against proton number, stable nuclei all lie close to a line. On the figure above, sketch this line. [1] Mr Powell 2013 3 Mini Progress Check – Self, Peer or Teacher Assess (3) c) Zirconium-100 decays initially to niobium-100. data: (i) nuclear masses: zirconium-100 niobium-100 electron mass 99.895 808 u 99.891 679 u 0.000 549 u Calculate the mass defect for this decay reaction. mass defect = ...................................................... u [2] (ii) Show that this mass defect is equivalent to about 5 × 10–13 J. [2] (iii) When a particular zirconium-100 nucleus decays, the emitted – particle has only about 2 × 10–13 J. Suggest why this is less than the energy calculated in (ii). ............................................................................................................... ............................................................................................................... ............................................................................................................... ............................................................................................................... [2] [Total 12 marks) Mr Powell 2013 4 Mini Progress Check – Self, Peer or Teacher Assess Answers 1) Any seven from: α - particle scattering suitable diagram with source, foil, moveable detector 2 or more trajectories shown vacuum most particles have little if any deflection large deflection of very few reference to Coulomb’s law /elastic scattering alphas repelled by nucleus (positive charges) monoenergetic OR electron scattering High energy diagram with source sample, moveable detector / film Vacuum Electron accelerator or other detail Most have zero deflection Characteristic angular distribution with minimum Minimum not zero De Broglie wavelength Wavelength comparable to nuclear size hence high energy B1 × 7 Clearly shows how evidence for the size of the nucleus follows from what is described. (1) [8] 2) acts only on nearest neighbour / when nuclei are 1 diameter apart; (1) either so force holding nucleons/ neutrons together independent of size of nucleus (1) or reference to b so distance apart (of nucleons) must be constant; so density of nucleus is independent of size; (1) 3 [3] Mr Powell 2013 5 Mini Progress Check – Self, Peer or Teacher Assess #3) (a) (b) (c) 236 U → 100 Zr + 131 Te + 51 n (1) 92 40 52 0 (i) nucleon number: no change proton number: increases by (1) 1 1 (ii) nucleon number: 100 proton number: 44 (1) 1 (iii) 5 correct points (1) 4 correct arrows (1) 2 (iv) straight line through / close to 56 / 44 of (1) ≤ gradient < 2 if curved, correct sense (1) 1 (i) reactant mass = 99.895 808 u product mass = 99.891 679 + 0.000 549 (= 99.892 228 u) (1) mass defect = 0.003 580 u (1) 2 (ii) Δm = 0.003 580 × 1.66 × 10–27 (= 5.943 × 10–30 kg) (1) E = (Δ)m c2 = 5.943 × 10–30 × (3.0 × 108)2 (= 5.35 × 10–13 J) (1) 2 or uses 1 u = 931 MeV so 0.00358 = 931 × 0.00358 (= 3.33 MeV) (1) = 3.33 × 1.6 × 10–13 (= 5.33 × 10–12 J) (1) (iii) (anti-)neutrino is also emitted (1) (anti-)neutrino has (some) energy (1) recoiling (niobium) nucleus has (kinetic) energy (1) any 2 2 [12] Mr Powell 2013 6