Pascal's Principle & Boyle's Law Worksheet
advertisement

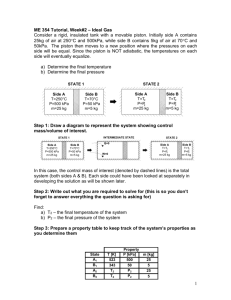
Pascal’s Principle F1 F2 A1 A2 1. A dentist’s chair makes use of Pascal’s principle to move patients up and down. Together, the chair and a patient exert a downward force of 2269 N. The chair is attached to a large piston with an area of 1,221 cm2. To move the chair, a pump applies force to a small piston with an area of 88.12 cm2. What force must be exerted on the small piston to lift the chair? 2. A hydraulic lift office chair has its seat attached to a piston with an area of 11.2 cm2. The chair is raised by exerting force on another piston, with an area of 4.12 cm2. If a person sitting on the chair exerts a downward force of 219 N, what force needs to be exerted on the small piston to lift the seat? 3. In changing a tire, a hydraulic jack lifts 7,468 N on its large piston, which has an area of 28.27 cm2. How much force must be exerted on the small piston if it has an area of 1.325 cm2? 4. An engine shop uses a lift to raise a 1,784 N engine. The lift has a large piston with an area of 76.32 cm2. To raise the lift, force is exerted on a small piston with an area of 12.56 cm2. What force must be exerted to raise the lift? 5. An engineering student wants to build her own hydraulic pump to lift a 1,815 N crate. The pump will have two pistons connected via a fluid chamber. The student calculates that she will be able to exert 442 N of force on the small piston, which will have an area of 50.2 cm2. What area must the large piston be to exert the desired force? 6. In a newly designed car with a hydraulic braking system, a force of 85 N is applied to one of the master cylinders, which has an area of 8.1 cm2. The master cylinder is connected to one brake piston, which exerts a force of 296 N. What is the area of the brake piston? 7. A mechanic uses a hydraulic car jack to lift the front end of a car to change the oil. The jack she uses exerts 8,915 N of force from the larger piston. To pump the jack, she exerts 444 N of force on the small piston, which has an area of 3.14 cm2. What is the area of the large piston? 8. A student in the lunchroom blows into his straw with a force of 0.26 N. The column of air pushing the liquid in the glass has an area of 0.21 cm2. If the liquid in the glass pushes upward with a force of 79 N, what is the area of the liquid at the surface of the glass? 9. The motor on a construction grade hydraulic shovel exerts 300,000 Pa of pressure on a fluid tank. The fluid tank is connected to a piston that has an area of 153 cm2. How much force does the piston exert? 10. A small crane has a motor that exerts 2.41 x 107 Pa of pressure on a fluid chamber. The chamber is connected by a fluid line to a piston on the crane arm. If the piston has an area of 168 cm2, how much force does the piston exert? 11. A bicycle pump uses Pascal’s law to operate. The air in the hose acts as a fluid and transfers force and pressure from the piston to the tire. If a pump has a piston with an area of 7.1 cm2, how much force must be exerted on it to create a pressure of 8.2 x 105 Pa? 12. A small, backyard log splitter has an engine that applies 1.723 x 107 Pa of pressure to a fluid tank. The tank is connected to piston with an area of 81.07 cm2. How much force can the piston exert? 13. A force of 38.7 N is applied to the master cylinder of a hydraulic brake system. The cylinder has an area of 7.61 cm 2. The force from the master cylinder is transferred, by brake fluid, to two brake cylinders that have a total area of 49.1 cm2. How much total force is exerted by the brake cylinders? 14. A factory lift is used to raise a load of 2,225 N on a piston that has an area of 706.8 cm2. How much pressure does the lift’s engine need to exert on the hydraulic fluid to lift the required load? Boyle’s Law P1V1 P2V2 1. To make an air horn, 1.50 L of air at 101 kPa are compressed into a can with a volume of 0.462 L. Assuming a constant temperature, what is the pressure on the compressed air? 2. A science class puts a balloon containing 1.25 L of air at 101 kPa into a bell jar. Using an air pump, the class removes some of the air in the jar, causing the balloon to expand to a volume of 2.25 L. Assuming a constant temperature, what is the new pressure inside the jar? 3. A small balloon is inflated with helium at 102 kPa to a volume of 2.12 L. According to the balloon’s manufacturer, if the balloon is stretched to a volume of 4.25 L, the balloon will pop. If the balloon were released, at what pressure would the balloon pop? Assume constant temperature throughout. 4. An oxygen supplier wants to reduce the volume of her oxygen tanks. She plans to take the oxygen in her 155 L tanks and store it in 95.5 L tanks. If the oxygen in the old tanks has a pressure of 8.27 x 103 kPa, what will the new pressure be after the oxygen is compressed? Assume a constant temperature throughout. 5. A blocked bicycle pump contains 0.682 L of air at 99.3 kPa. If the handle is pressed down, decreasing the volume of the inside air to 0.151 L, what is the pressure inside the pump? Assume that the temperature of the air does not change. 6. A balloon is filled with air at a pressure of 105 kPa. Then the pressure around the balloon is increased to 205 kPa. If the balloon originally had a volume of 4.11 L, what is the new volume of the balloon? Assume constant temperature throughout. 7. An oxygen tank holds 355 L of oxygen at 8.23 x 103 kPa. What volume would the same amount of oxygen take up if the pressure were reduced to 4.11 x 103 kPa? Assume that the temperature remains the same. 8. A machine produces 599 L of hydrogen at 101 kPa each day. If each day’s supply of hydrogen were kept at a pressure of 366 kPa, what would be the volume of the hydrogen? Assume that temperature is constant throughout. 9. A diver’s tank holds 15.1 L of air at a pressure of 1.53 x 104 kPa. If the air was released underwater, at a pressure of 6.11 x 102 kPa, what would the volume of the released air be? Assume that temperature is constant throughout. 10. A plastic food storage bag is sealed with 0.213 L of air inside at a pressure of 99.2 kPa. The bag is loaded onto a plane, where the pressure is decreased to 80.5 kPa. What is the size of the air in the bag after the pressure is decreased, if the temperature of the air remains the same? 11. A balloon is filled with helium in an airplane, where the air pressure is 81 kPa. The filled balloon has a volume of 4.2 L When the plane lands, the cabin is depressurized to the outside air pressure, which is 94 kPa. If the temperature of the balloon remains the same, what is the new volume of the balloon? 12. A small helium tank claims to be able to fill 30 balloons to a volume of 3.15 L at a pressure of 101 kPa. How many liters of helium will the tank be able to produce at a pressure of 94.2 kPa? Assume that the temperature of the tank remains the same throughout. 13. A scuba diver carries small balloon of air deep underwater. If the balloon has a volume of 2.11 L at the surface, where the pressure is 102 kPa, what will the pressure be when the balloon has shrunk to a size of 0.581 L? Assume that the balloon’s temperature remains the same throughout its decent. 14. A medical supply company stores oxygen in a 200.5 L tank at a pressure of 9,585 kPa. If the company transfers all of the oxygen to a 350.8 L tank, what will the pressure be inside the tank, if the temperature stays the same? 15. At a sewage treatment plant, methane is gathered for energy use. If 75 L of methane is produced at 94 kPa, how many liters would be produced at 100 kPa? Assume temperature remains constant throughout.