nph13064-sup-0001-FigS1-S2
advertisement

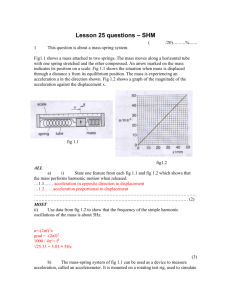
New Phytologist Supporting Information Figs S1 & S2, Notes S1 and legend to Video S1 Article title: Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature Authors: Kathrin Thor and Edgar Peiter Article acceptance date: 16 August 2014 The following Supporting Information is available for this article: Fig. S1 Effects of inhibitors of GLRs and CNGCs on flg22-induced [Ca2+]cyt oscillations in stomatal guard cells. Fig. S2 Analysis of TMB-8 effects on guard cell [Ca2+]cyt. Notes S1 Aequorin based [Ca2+]cyt determination. Video S1 Time-lapse movie of flg22-induced [Ca2+]cyt responses in Arabidopsis guard cells. 1 Fig. S1 Effects of inhibitors of GLRs and CNGCs on flg22-induced [Ca2+]cyt oscillations in stomatal guard cells. (a) AP-5, a competitive antagonist of the Glu-binding site of NMDA-type channels, has no marked effect. In general, cells exhibited oscillations after addition of flg22 with a pattern resembling that obtained without inhibitor. Shown are two representative traces, one exhibiting oscillations of very high frequency shortly after flg22-addition, another one exhibiting slightly less frequent spiking. Numbers indicate the proportion of cells showing a similar pattern, when cells were incubated for 5 min (upper digits) or 1 h (lower digits) in buffer containing 5 mM AP-5 before starting the measurement. The proportion of cells exhibiting the slightly less frequent spiking was somewhat increased by the 1 h inhibitor pre-incubation. (b) Kynurenic acid, an inhibitor of both, NMDA- and non-NMDA-type receptors, mainly affects the duration of the oscillations, which is reflected by a reduced number of peaks (Fig. 3). Shown are three representative traces. Cells were incubated in buffer containing 1 mM kynurenic acid for 5 min prior to starting the experiment. As kynurenic acid is targeting both NMDA and nonNMDA receptors in animals, and as AP-5 had no clear effect, it could be speculated that the later spikes, occurring after around 15 minutes of flg22 exposure, might be provoked by nonNMDA-like GLRs. However, DNQX, which targets non-NMDA glutamate receptors, did also not abolish the Ca2+ oscillations, although the spike number was somewhat reduced (Fig. 3). (c) Representative traces for cells incubated in 1 mM DNQX for 5 min (upper traces) or 1 h (lower traces) are shown. (d) 1 mM L-Glu has no desensitizing effect on the flg22-induced oscillations and itself induces a [Ca2+]cyt signal. Upper trace: Cells were incubated in incubation buffer containing 1 mM L-Glu for 1 h prior to starting the experiment. Middle trace: 1 mM L-Glu was added at time point 0. Lower trace: 1 mM L-Glu was added at time point 0 followed by 100 nM flg22 15 min later (indicated by an arrow). (e) Alloxan has no effect. Shown are representative traces of cells incubated in incubation buffer containing 1 mM alloxan for 5 min (upper trace) or 1 h (lower trace) prior to starting the measurement. (b-e) Each trace shows the response of one representative cell. Numbers indicate the proportion of cells showing a similar pattern. 2 Figure S1 3 Fig. S2 Analysis of TMB-8 effects on guard cell [Ca2+]cyt. (a) YFP and CFP fluorescence recordings for the upper trace in Fig. 2(f). The arrow indicates the ratio increase visible in Fig. 2(f) at around 15 min. (b) Oscillations observed in Fig 2(f) result from TMB-8 rather than flg22. Upper trace: TMB-8 was applied 5 min before starting the experiment. Middle trace: TMB-8 was added at the beginning of the measurement (indicated by an arrow). Lower trace: TMB-8 was added 10 min after starting the measurement (time point -5 min). flg22, which is normally applied at time point 0, was not applied in any of the experiments. Each line shows the response of one representative cell. Numbers indicate the proportion of cells showing a similar pattern. Figure S2 4 Notes S1 Aequorin based [Ca2+]cyt determination. Aequorin luminescence measurements on whole seedlings were performed in principle as described by Kwaaitaal et al. (2011). Briefly, plants grown in MS +0.25% sucrose in 96 well-plates were reconstituted overnight in 100 µl of a 10 µM coelenterazine solution. The next day, the solution was exchanged by 100 µl H 2O, and the plants were incubated for another hour in the dark. Measurements were performed using a Mithras LB 940 microplate reader (Berthold, Bad Wildbad, Germany). 50 µl flg22 solution were injected to yield a concentration of 100 nM. For aequorin discharging, 100 µl of a solution containing 2 M CaCl2 and 20% ethanol (CaEt) were injected. For aequorin measurements on epidermal strips, ten mature leaves were blended in 55 ml incubation buffer for 30 s at low speed (8011EG with MC-2 container, Waring, Torrington, CT, USA). The suspension was filtered through a 70 µm nylon filter (BD, Franklin Lakes, NJ, USA). Strips remaining on the filter were washed several times with incubation buffer and transferred into 35 mm petri dishes containing 2 ml of incubation buffer and 10 µM coelenterazine. Strips were incubated overnight at room temperature in the dark. The following morning, strips were transferred into a luminometer tube containing 200 µl incubation buffer and incubated in the dark for another 2 h. Aequorin luminescence was detected using a modified Sirius-2 tube luminometer (Titertek-Berthold, Pforzheim, Germany). Flg22 was injected as 10x stock to yield a final concentration of 100 nM. Incubation buffer was injected as control. For aequorin discharging, 200 µl of CaEt were injected. [Ca2+]cyt in seedlings and epidermes was calculated according to Rentel & Knight (2004). References Kwaaitaal M, Huisman R, Maintz J, Reinstädler A, Panstruga R. 2011. Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochemical Journal 440: 355-365. Rentel MC, Knight MR. 2004. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiology 135: 1471-1479. 5 Video S1 Time-lapse movie of flg22-induced [Ca2+]cyt responses in Arabidopsis guard cells. Flg22 (100 nM) was added at T=0. The ratio of YFP-to-CFP fluorescence intensity of the Ca2+ reporter YC3.6 is colour-coded according to the scale shown in Fig. 1(d), with blue indicating lowest and white indicating highest [Ca2+]cyt. Time is given as hh:mm:ss. 6
![Substantiation of the Rhod-2 as indicator of cytosolic [Ca2+] Rhod](http://s3.studylib.net/store/data/006893824_1-225923ad9f8cdb438dcdcf307ccbe9bd-300x300.png)