Superficial bladder cancer is rarely found during a - Dis Lair
advertisement

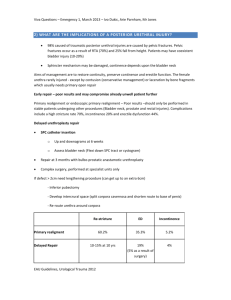
Bladder Cancer Introduction Background Bladder cancer is a common urologic cancer. The most common type of bladder cancer in the United States is urothelial carcinoma, formerly known as transitional cell carcinoma (TCC). The urothelium in the entire urinary tract may be involved, including the renal pelvis, ureter, bladder, and urethra. The clinical course of bladder cancer carries a broad spectrum of aggressiveness and risk. Low-grade, superficial bladder cancers have minimal risk of progression to death; however, high-grade muscle-invasive cancers are often lethal. Pathophysiology Almost all bladder cancers are epithelial in origin. The urothelium consists of a 3- to 7-cell mucosal layer within the muscular bladder. Of these urothelial tumors, more than 90% are transitional cell carcinomas. However, up to 5% of bladder cancers are squamous cell in origin, and 2% are adenocarcinomas. Nonurothelial primary bladder tumors are extremely rare and may include small cell carcinoma, carcinosarcoma, primary lymphoma, and sarcoma. Bladder cancer is often described as a polyclonal field change defect with frequent recurrences due to a heightened potential for malignant transformation. However, bladder cancer has also been described as a problem with implantation and migration from a previously affected site. The World Health Organization classifies bladder cancers as low grade (grade 1 and 2) or high grade (grade 3). Tumors are also classified by growth patterns: papillary (70%), sessile or mixed (20%), and nodular (10%).Carcinoma in situ (CIS) is a flat, noninvasive, high-grade urothelial carcinoma. The most significant prognostic factors for bladder cancer are grade, depth of invasion, and the presence of CIS. Upon presentation, 55-60% of patients have low-grade superficial disease, which is usually treated conservatively with transurethral resection and periodic cystoscopy. Forty to forty-five percent of patients have high-grade disease, of which 50% is muscle invasive and is typically treated with radical cystectomy. Less than 5% of bladder cancers in the United States are squamous cell carcinomas (SCCs). However, worldwide, SCC is the most common form, accounting for 75% of bladder cancer in underdeveloped nations. In the United States, SCC is associated with persistent inflammation from long-term indwelling Foley catheters and bladder stones. In underdeveloped nations, SCC is associated with bladder infection by Schistosoma haematobium. Adenocarcinomas account for less than 2% of primary bladder tumors. These tumors are observed most commonly in exstrophic bladders and respond poorly to radiation and chemotherapy. Radical cystectomy is the treatment of choice. Small cell carcinomas are aggressive tumors associated with a poor prognosis and are thought to arise from neuroendocrine stem cells. Carcinosarcomas are highly malignant tumors that contain both mesenchymal and epithelial elements. Primary bladder lymphomas arise in the submucosa of the bladder and are treated with radiation therapy. Leiomyosarcoma is the most common sarcoma of the bladder. Rhabdomyosarcomas most commonly occur in children and carry a poor prognosis. Frequency United States Bladder cancer is the fourth most common cancer in men in the United States, after prostate, lung, and colorectal cancer. Bladder cancer is the 10th most common cancer in women. From 1985-2000, the number of patients diagnosed annually with bladder cancer increased by 33%. An annual cohort of 300,000-400,000 patients with bladder cancer is reported in the United States. The recurrence rate for superficial transitional cell cancer of the bladder is high, and as many as 80% of patients have at least one recurrence. International In developed countries, 90% of bladder cancers are TCC. In developing countries, 75% of bladder cancers are SCCs, and most of these cancers are secondary to S haematobium infection. Mortality/Morbidity In 2009, an estimated 70,980 new patients will be diagnosed with bladder cancer in the United States, and 14,330 of those patients will die of the disease.1 Race Bladder cancer is more common in whites than in blacks; however, blacks have a worse prognosis than whites. Sex The male-to-female ratio is 3:1. Women generally have a worse prognosis than men. Age The median age at diagnosis is 68 years, and the incidence increases with age. Clinical History 1. Approximately 80-90% of patients with bladder cancer present with painless gross hematuria, which is the classic presentation. Consider all patients with gross hematuria to have bladder cancer until proven otherwise. Suspect bladder cancer if any patient presents with unexplained microscopic hematuria. 2. Twenty to thirty percent of patients with bladder cancer experience irritative bladder symptoms such as dysuria, urgency, or frequency of urination that are related to more advanced muscle-invasive disease or CIS. 3. Patients with advanced disease can present with pelvic or bony pain, lower-extremity edema from iliac vessel compression, or flank pain from ureteral obstruction. Physical Superficial bladder cancer is rarely found during a physical examination. Occasionally, an abdominal or pelvic mass may be palpable. Examine for lymphadenopathy. Causes Up to 80% of bladder cancer cases are associated with environmental exposure, which suggests that bladder cancer is potentially preventable. Smoking is the most commonly associated risk factor and accounts for approximately 50% of all bladder cancers. Nitrosamine, 2-naphthylamine, and 4aminobiphenyl are possible carcinogenic agents found in cigarette smoke. Bladder cancer is also associated with industrial exposure to aromatic amines in dyes, paints, solvents, leather dust, inks, combustion products, rubber, and textiles. Therefore, higher-risk occupations associated with bladder cancer include painting, driving trucks, and working with metal. Several medical risk factors are associated with bladder cancer. Patients with prior exposure to radiation treatment of the pelvis have an increased risk of bladder cancer. Chemotherapy with cyclophosphamide increases the risk of bladder cancer via exposure to acrolein, a urinary metabolite of cyclophosphamide. Patients with spinal cord injuries who have long-term indwelling catheters have a 16- to 20-fold increased risk of developing SCC of the bladder. Coffee consumption does not increase the risk of developing bladder cancer. Early studies of rodents and a minority of human studies suggested a weak connection between artificial sweeteners (eg, saccharin, cyclamate) and bladder cancer; however, most recent studies show no significant correlation. Although no convincing evidence exists for a hereditary factor in the development of bladder cancer, familial clusters of bladder cancer have been reported. Several genetic mutations have been identified in bladder cancer. Mutations of the tumor suppressor gene for p53, found on chromosome 17, are associated with high-grade bladder cancer and CIS. Mutations of the tumor suppressor gene for p15 and p16, found on chromosome 9, are associated with low-grade and superficial tumors. Retinoblastoma (Rb) tumor suppressor gene mutations are also noted. Bladder cancer is associated with increased expression of the epidermal growth factor gene and the erb- b2 oncogene, and mutations of the oncogenes p21 ras, c-myc, and c-jun. Differential Diagnoses Hemorrhagic Noninfectious Cystitis: Urinary Females Nephrolithiasis Tract Urinary Tract Infection, Males Renal Cell Carcinoma Transitional Cell Carcinoma, Renal Infection, Ureteral Trauma Workup Laboratory Studies 1. Any patient with gross or microscopic hematuria should be urologically evaluated. Microscopic hematuria from bladder cancer may be intermittent; therefore, a repeat negative result on urinalysis does not exclude the diagnosis. Infection may cause hematuria and is usually associated with irritative voiding symptoms (eg, dysuria, frequency, urgency). Irritative voiding symptoms may also be caused by CIS or muscle-invasive bladder cancer. Further evaluate irritative voiding symptoms caused by a urinary tract infection that do not resolve with treatment. Urinalysis with microscopy Urine culture to rule out infection, if suspected Voided urinary cytology (This may be helpful if results are positive, but a negative cytology result cannot be considered definitive. Urinary cytology for routine screening is controversial.) 2. Newer molecular and genetic markers may help in the early detection and prediction of urothelial carcinoma. Newer, voided urine assays (ie, bladder tumor antigen [BTA-Stat, BTA-TRAK], nuclear matrix protein [NMP-22], fibrin/fibrinogen degradation products [FDP]) are being used for the detection and surveillance of urothelial carcinoma. These tests have high false-positive and falsenegative rates. In the future, other newer assays based on telomerase and microsatellite analysis may prove to be a better detection method than urinary cytology. Chromosomal alterations have been associated with urothelial carcinoma. One encouraging test is a multitarget interphase fluorescence in situ hybridization (FISH) assay called UroVysion that consists of probes to the centromeres of chromosomes 3, 7, 17, and the 9p21 region. Aneuploidy of chromosomes 3, 7, and 17 and deletion of chromosome 9 has been associated with high sensitivity and specificity to detect bladder cancer. Often, this is an anticipatory positive result with a positive finding preceding visual evidence of bladder tumor. However, at this time, no urinary assay has been shown to effectively replace cystoscopy for the detection of bladder tumors. Imaging Studies 1. Upper-tract imaging is necessary for the hematuria workup and should be able to visualize both the kidneys and the urothelium. 2. The American Urologic Association Best Practice Policy recommends CT scanning of the abdomen and pelvis with preinfusion and postinfusion phases. This is ideally performed with a CT urography or followed by radiography of the kidneys, ureters, and bladder (KUB) to obtain images similar to those produced with intravenous pyelography (IVP). 3. Two commonly used alternative studies are IVP and renal ultrasonography. The IVP is the traditional standard for upper-tract urothelium imaging; however, it is poor for evaluating the renal parenchyma. Ultrasonography is also commonly used; however, urothelial tumors of the upper tract and small stones are easily missed. 4. Conduct retrograde pyelography in patients in whom contrast CT scanning cannot be performed because of azotemia or a severe allergy to intravenous contrast. Procedures 1. Cystoscopy Obtain biopsy samples of suspicious lesions during cystoscopy. Attempt to include the bladder muscle in the biopsy specimen. This allows the pathologist to determine whether the tumor is muscle invasive. Transitional cell tumors are typically papillary or sessile, and CIS may appear as an erythematous, velvety lesion. Unless the lesion is in a bladder diverticulum (pseudodiverticulum), attempt to resect the primary tumor completely. A bladder diverticulum lacks a surrounding muscle layer, and a deep biopsy of a lesion within a diverticulum risks perforating the bladder and extravesical extravasation of cancer cells. Because no muscle layer surrounds the bladder diverticulum, the next step in the progression of a superficial tumor is extravesical spread, requiring more aggressive surgical therapy (eg, partial cystectomy, open diverticulectomy) rather than a simple resection followed by surveillance. Further investigate efflux of blood from either ureteral orifice with retrograde pyelography, ureteroscopy, or both. 2. Urine cytology Perform urine cytology at the same time as cystoscopy, although its routine use for screening is controversial. Urine cytology is associated with a significant falsenegative rate, especially for low-grade carcinoma (1050% accuracy rate). The false-positive rate is 1-12%, but it has a 95% accuracy rate for diagnosing high-grade carcinoma and CIS. The sensitivity of urine cytology can be increased by obtaining a bladder barbotage cytology (70%) as opposed to a voided cytology (30%). With a normal finding on cystoscopic examination, further evaluate a positive cytology result on urine study with an upper-tract study and random biopsies of the bladder. Obtain biopsy samples of the prostatic urethra in men. 3. Other urine markers for bladder cancer The use of additional urine markers such as UroVysion (FISH), BTA, and NMP-22 in the initial diagnosis of bladder cancer is controversial. All of these assays may yield false-positive and false-negative results. These other tests should not replace urine cytology and cystoscopy, with or without biopsy, for the diagnosis of bladder cancer. However, they may be useful adjuncts to urine cytology and cystoscopy. Histologic Findings More than 90% of bladder cancer cases are TCC, approximately 5% are SCC, and less than 2% are adenocarcinoma. Both the stage and tumor grade correlate independently with prognosis. Staging The International Union Against Cancer and the American Joint Committee on Cancer Staging developed the tumor, node, and metastases (TNM) staging system, which is used to stage bladder cancer (see below). Ta and T1 tumors and CIS were once considered superficial bladder tumors. T2, T3, and T4 tumors were traditionally described as invasive bladder cancer. However, urologic oncologists now recommend avoiding the term superficial bladder cancer to describe Ta, T1, and CIS tumors because it is a misnomer and tends to group patients who may require different treatments and who may have differing prognoses. Urothelial carcinoma is histologically graded as low grade (formerly graded 1-2) or high grade (formerly graded 3). CIS is characterized by full mucosal thickness and high-grade dysplasia of the bladder epithelium and is associated with a poorer prognosis. The following is the TNM staging system for bladder cancer: 1. CIS - Carcinoma in situ, high-grade dysplasia, confined to the epithelium 2. Ta - Papillary tumor confined to the epithelium 3. T1 - Tumor invasion into the lamina propria 4. T2 - Tumor invasion into the muscularis propria 5. T3 - Tumor involvement of the perivesical fat 6. T4 - Tumor involvement of adjacent organs such as prostate, rectum, or pelvic sidewall 7. N+ - Lymph node metastasis 8. M+ - Metastasis More than 70% of all newly diagnosed bladder cancers are non–muscle invasive, approximately 50-70% are Ta, 20-30% are T1, and 10% are CIS. Approximately 5% of patients present with metastatic disease, which commonly involves the lymph nodes, lung, liver, bone, and central nervous system. Approximately 25% of affected patients have muscleinvasive disease at diagnosis. 1. Clinically stage a patient who has muscle-invasive disease with CT scanning of the abdomen and pelvis, chest radiography, and serum chemistries. 2. If the patient is asymptomatic with normal calcium and alkaline phosphatase levels, a bone scan is unnecessary. 3. As many as 50% of patients with muscle-invasive bladder cancer may have occult metastases that become clinically apparent within 5 years of initial diagnosis. 4. Most patients with overt metastatic disease die within 2 years despite chemotherapy. 5. Approximately 25-30% of patients with only limited regional lymph node metastasis discovered during cystectomy and pelvic lymph node dissection may survive beyond 5 years. Treatment Medical Care The treatment of non–muscle-invasive (Ta, T1, CIS) and muscle-invasive bladder cancer should be differentiated. Treatments within each category include both surgical and medical approaches. 1. Non–muscle-invasive disease (Ta, T1, CIS) a) Intravesical immunotherapy (Bacillus Calmette-Guérin [BCG] immunotherapy) BCG immunotherapy is used in the treatment of Ta, T1, and CIS urothelial carcinoma of the bladder and may help to decrease the rate of recurrence and progression. BCG immunotherapy is the most effective intravesical therapy and involves a live attenuated strain of Mycobacterium bovis.Some early studies purported that an immune response against BCG surface antigens cross-reacted with putative bladder tumor antigens, and this was proposed as the mechanism for the therapeutic effect of BCG; however, multiple subsequent studies refute this claim and demonstrate that BCG induces a nonspecific, cytokine-mediated immune response to foreign protein. Because BCG is a live attenuated organism, it can cause an acute disseminated tuberculosislike illness if it enters the bloodstream (BCG sepsis), possibly resulting in death. Therefore, the use of BCG is contraindicated in patients with gross hematuria. BCG typically causes mild systemic symptoms that resolve within 24-48 hours after intravesical instillation. BCG can also cause granulomatous cystitis or prostatitis with bladder contraction. BCG is recommended for CIS, T1 tumors, and high-risk Ta tumors (large, high-grade, recurrent, or multifocal tumors). This therapy is less effective in reducing the 5year recurrence rate for low-grade and low-stage urothelial carcinoma (see Table 1 below). Typically, BCG is administered weekly for 6 weeks. Another 6-week course may be administered if a repeat cystoscopy reveals tumor persistence or recurrence. Recent evidence indicates that maintenance therapy with a weekly treatment for 3 weeks every 6 months for 1-3 years may provide more lasting results. Consider patients with recurrent CIS for an early cystectomy. At 5 and 10 years, approximately 70% and 30% of patients with CIS who are treated with BCG are disease free, respectively. Recurrent CIS, despite intravesical BCG, is associated with a 63% risk of progression to muscle-invasive bladder cancer. Recurrence after BCG treatment may also occur in the upper urinary tract or prostatic urethra. Interferon alpha or gamma has been used in the treatment of stages Ta, T1 and CIS urothelial carcinoma, either as a single agent therapy or in combination with BCG. Its role has primarily been in post-BCG failure with early promising results. Although BCG with interferon has shown a 42% response with tolerable side effects after BCG failure, no evidence has indicated that retreating with BCG with interferon is superior to retreating with BCG alone. b) Intravesical chemotherapy Valrubicin has recently been approved as intravesical chemotherapy for CIS that is refractory to BCG. In patients whose conditions do not respond to BCG, the overall response rate to valrubicin is approximately 20%, and some patients can delay time to cystectomy. Valrubicin is presently not commercially available. Other forms of adjuvant intravesical chemotherapy for bladder cancer include intravesical triethylenethiophosphoramide (thiotepa [Thioplex]), mitomycin-C, doxorubicin, and epirubicin. Although these agents may increase the time to disease recurrence, no evidence indicates that these therapies prevent disease progression. No evidence suggests that these adjuvant therapies are as effective as BCG. 2. Muscle-invasive disease (T2 and greater) a) Adjuvant and neoadjuvant chemotherapy Neoadjuvant chemotherapy prior to either radical cystectomy or external beam radiotherapy is controversial. The Southwestern Oncology Group (SWOG) conducted a multicenter randomized prospective study that compared neoadjuvant therapy using a methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (MVAC) combination with surgery alone. The group concluded that neoadjuvant therapy conferred a treatment benefit compared with surgery alone. However, several criticisms of this study exist. The study was purposely underpowered because of slow recruitment (317 patients over 11 y), because 20% of the patients who were to undergo cystectomy alone never underwent surgery, and because there was no comparison to neoadjuvant therapy alone. In addition, a recent study re-evaluated the SWOG data and found that surgical factors significantly affected outcomes. In one small series, the T4 tumors of 45% of affected patients responded to chemotherapy, making potentially curative cystectomy possible. Although no definite evidence of benefit exists, patients with P3-P4 or N+ urothelial carcinoma in the United States are typically advised to receive adjuvant chemotherapy. b) Chemotherapeutic agents for metastatic disease MVAC is the standard treatment of metastatic bladder cancer. MVAC has an objective response rate of 57-70%, a complete response rate of 15-20%, and a 2-year survival rate of 15-20%. Gemcitabine and cisplatin (GC) is a newer regimen and has been shown to be as efficacious as MVAC, but with less toxicity. GC is now considered a first-line treatment agent for bladder cancer. Several novel compounds have shown activity against transitional cell bladder cancer and are now being tested in combination chemotherapy trials. Some of these promising agents are ifosfamide, paclitaxel, docetaxel, and carboplatin. Table 1. Recurrence and Progression Rates at 5 Years for Ta, T1, and CIS TCC of the Bladder Treated With BCG Stage Recurrence, % Progression, % Ta 55 11 T1 61 31 CIS 45 23 G1 61 7 G2 56 19 G3 45 23 Surgical Care 1. Ta, T1, and CIS a) Endoscopic treatment Transurethral resection of bladder tumor (TURBT) is the first-line treatment to diagnose, to stage, and to treat visible tumors. Patients with bulky, high-grade, or multifocal tumors should undergo a second procedure to ensure complete resection and accurate staging. Approximately 50% of stage T1 tumors are upgraded to muscle-invasive disease. Electrocautery or laser fulguration of the bladder tumor is sufficient for low-grade, small-volume, papillary tumors. No further metastatic workup is needed for obviously superficial tumors. Because bladder cancer is a polyclonal field change defect, continued surveillance is mandatory. b) Radical cystectomy Although typically reserved for muscle-invasive disease, radical surgery is more appropriately used to treat some cases of non–muscle-invasive bladder cancer. Thirty-five to fifty percent of patients who undergo cystectomy for Ta, T1, or CIS are discovered to have muscle-invasive disease, with 10-15% demonstrating microscopic lymph node metastasis. The CIS in upwards of 80% of affected patients progresses to muscle-invasive disease, with 20% of patients found to have muscle-invasive disease at the time of cystectomy. High-grade T1 tumors that recur despite BCG have a 50% likelihood of progressing to muscle-invasive disease. Cystectomy performed prior to progression yields a 90% 5-year survival rate. The 5-year survival rate drops to 5060% in muscle-invasive disease. Patients with unresectable large superficial tumors, prostatic urethra involvement, and BCG failure should also undergo radical cystectomy. In 2009, the Society of Urologic Oncology (SUO) discussed the current status of robot-assisted radical cystectomy (RARC). Experienced surgeons demonstrated the possibility of reduced blood loss, opiate requirement, and hospital stay. Surgeons performing this procedure need to provide detailed informed consent and a full description of potential complications and outcomes when confronting a serious disease with profound postoperative quality-of-life changes, as this is a relatively new procedure.2 2. Muscle-invasive disease (T2 and greater) a) Radical cystoprostatectomy (men) In men, this is the criterion standard for organ-confined, muscle-invasive bladder cancer (eg, T2, T3). Remove the bladder, prostate, and pelvic lymph nodes. Perform a total urethrectomy for anterior urethral involvement, involvement of the prostatic stroma, or diffuse CIS that involves the prostate. b) Anterior pelvic exenteration (women) Perform this procedure in women diagnosed with muscle-invasive bladder cancer. The procedure involves removal of the bladder, urethra, uterus, ovaries, anterior vaginal wall, and pelvic lymph nodes. If no tumor involvement of the bladder neck is present, the urethra and anterior vaginal wall may be spared with the construction of an orthotopic neobladder. c) Pelvic lymphadenectomy Approximately 25% of patients undergoing radical cystectomy have lymph node metastases at the time of surgery. Bilateral pelvic lymphadenectomy (PLND) should be performed in conjunction with radical cystoprostatectomy and anterior pelvic exenteration. PLND adds prognostic information by appropriately staging the patient and may confer a therapeutic benefit. The boundaries of a standard PLND include the bifurcation of the common iliac artery and vein superiorly, the genitofemoral nerve laterally, the obturator fossa posteriorly, and the circumflex iliac vein (or node of Cloquet) inferiorly. Extended PLND includes the lymph nodes in the presacral region and those surrounding the common iliac vessels to the level of the aortic bifurcation. The additional benefit of an extended PLND is controversial. Based on several retrospective studies, some experts believe that an extended dissection provides additional staging information and offers a survival benefit. However, no randomized trials to date have proven that an extended PLND is more beneficial than a standard PLND. d) After performing a cystectomy, a urinary diversion must be created from an intestinal segment. The various types of urinary diversions can be separated into the following continent and incontinent diversions: Conduit (incontinent diversion; see image below): Conduits can be constructed from either ileum or colon. The ileal conduit is the most common incontinent diversion performed and has been used for more than 40 years with excellent reliability and minimal morbidity. A small segment of ileum (at least 15 cm proximal to the ileocecal valve) is taken out of gastrointestinal continuity but maintained on its mesentery, with care to preserve its blood supply. The gastrointestinal tract is restored with a small-bowel anastomosis. The ureters are anastomosed to an end or side of this intestinal segment and the other end is brought out as a stoma to the abdominal wall. Urine continuously collects in an external collection device worn over the stoma. In an ileal conduit, a small segment of ileum is taken out of continuity with the gastrointestinal tract but is maintained on its mesentery. Ureters are anastomosed to one end of this ileal segment, and the other end is brought out as a stoma to the abdominal wall. Indiana pouch (continent diversion; see image below): This is a continent urinary reservoir created from a detubularized right colon and an efferent limb of terminal ileum. The terminal ileum is plicated and brought to the abdominal wall. The ileocecal valve acts as a continence mechanism. The Indiana pouch is emptied with a clean intermittent catheterization 4-6 times per day. In an Indiana pouch, a urinary reservoir is created from detubularized right colon and an efferent limb of terminal ileum. Terminal ileum is plicated and brought to the abdominal wall. The continence mechanism is the ileocecal valve. Neobladder (continent diversion; see image below): Various segments of intestine including ileum, ileum and colon, and sigmoid colon can be used to construct a reservoir. The ureters are implanted to the reservoir, and the reservoir is anastomosed to the urethra. This operation has been performed successfully in men for more than 20 years and, more recently, in women. The orthotopic neobladder most closely restores the natural storage and voiding function of the native bladder. Patients have volitional control of urination and void by Valsalva. Contraindications to performing continent urinary diversions include multiple comorbid health problems, chronic renal insufficiency, hepatic dysfunction, and advanced disease stage. In an orthotopic neobladder, a segment of ileum is used to construct a neobladder, which is connected to the urethra. Orthotopic neobladder most closely restores the natural storage and voiding function of the native bladder. e) Laparoscopic and robotic surgery Recently, laparoscopic and robotic-assisted radical cystectomies have been performed in small numbers at select tertiary academic centers. The urinary diversion is almost universally performed extracorporeally through a miniature laparotomy incision. Initially, some centers attempted to create the urinary diversion laparoscopically, but this was abandoned because of inferior outcomes. 3 Immediate postoperative complication rates and functional outcomes appear to be similar to those of open radical cystectomy and urinary diversion. In addition, a few studies suggest faster recovery of bowel function and less use of postoperative narcotics. However, these findings have not been corroborated by other contemporary studies. Intermediate and long-term oncologic outcomes for these minimally invasive approaches remain undefined. At this time, open radical cystectomy and urinary diversion should be considered the standard of care for invasive bladder cancer, and patients should be counseled to this end. Both laparoscopic and robotic-assisted radical cystectomy remain investigative procedures that should be performed only at major academic medical centers after appropriate informed consent. Radiation therapy External beam radiation therapy has been shown to be inferior to radical cystectomy for the treatment of f) bladder cancer. The overall 5-year survival rate after treatment with external beam radiation is 20-40% compared to a 90% 5-year survival after cystectomy for organ-confined disease. Although inferior to radical cystectomy, external beam radiation therapy is used in various countries other than the United States for T2-T3 urothelial carcinoma of the bladder. Neoadjuvant external beam radiation therapy has been attempted for muscle-invasive bladder cancer, with no improvement in survival rate. g) In certain facilities, a bladder-preserving strategy for T2T3 urothelial carcinoma is applied using a combination of external beam radiation, chemotherapy, and endoscopic resection. Survival rates associated with this approach are comparable with those of cystectomy in selected patients. This combination has a widespread application that is limited by the complexity of the protocol, its toxicity, and a high mortality rate. The mortality rate in the 2 largest US series with the longest follow-up study is 4-5%. In comparison, the mortality rate for most modern cystectomy series is 12%. In addition, a significant number of patients ultimately require salvage cystectomy, which is associated with significantly increased morbidity and decreased options for urinary diversions. In some series, local recurrence of bladder cancer is as high as 50-60% despite the completion of bladder-preserving therapy. Medication MVAC is the standard treatment for metastatic bladder cancer. No proven role exists for adjuvant chemotherapy. When selecting therapy, the MVAC combination has substantial toxicity and must be weighed against the expected benefit. The major dose-limiting toxicity is myelosuppression. The new combination regimens (eg, gemcitabine, cisplatin) show response rates and median survival comparable to MVAC but with less toxicity. Antineoplastic agents These agents inhibit cell growth and proliferation. Methotrexate (Folex PFS) Inhibits dihydrofolate reductase (DHFR), causing a block in the reduction of dihydrofolate to tetrahydrofolate. This inhibits the formation of thymidylate and purines and arrests DNA, RNA, and protein synthesis. Adult 30 mg/m2 IV on day 1; repeat on days 15 and 22 if WBC count >2000/µL and platelet count >50,000/µL Vinblastine (Velban, Alkaban-AQ) Vinca alkaloid with cytotoxic effect via mitotic arrest. Binds to specific site on tubulin, prevents polymerization of tubulin dimers, and inhibits microtubule formation. Intrathecal (IT) administration use may result in death. Adult 3 mg/m2 IV on day 2; repeat on days 15 and 22 if WBC count >2000/µL and platelet count >50,000/µL Doxorubicin (Adriamycin) Anthracycline antibiotic that causes DNA strand breakage through effects on topoisomerase II and direct intercalation into DNA, which causes DNA polymerase inhibition. This drug is both mutagenic and carcinogenic. Adult 30 mg/m2 IV on day 2 Cisplatin (Platinol) A platinum-containing compound that exerts an antineoplastic effect by covalently binding to DNA, with preferential binding to N-7 position of guanine and adenosine. Can react with 2 different sites on DNA to produce cross-links. Platinum complex also can bind to nucleus and cytoplasmic protein. Adult 50-70 mg/m2 IV on day 2 Gemcitabine (Gemzar) Cytidine analog. After intracellular metabolism to active nucleotide, inhibits ribonucleotide reductase and competes with deoxycytidine triphosphate for incorporation into DNA. Adult 1 g/m2 IV Follow-up Further Outpatient Care The high rate of disease recurrence and progression in non– muscle invasive bladder cancer underscores the need for careful follow-up studies. Surveillance for these patients includes cystoscopy and bladder wash cytologies every 3 months for 2 years, then every 6 months for 2 years, and then at least yearly. Cystoscopy Cystoscopy is the primary diagnostic modality for the diagnosis of bladder carcinoma because it confers low risk and can be performed in the physician's office. Although it is the criterion standard for detecting bladder cancer, cystoscopy is invasive and relatively expensive.4 Moreover, visibility can be reduced by bleeding, and flat urothelial lesions such as CIS may be difficult to distinguish from normal bladder tissue. Thus, cytologic analysis of voided urine is frequently used as an adjunctive test to aid in identifying occult cancers. Cytology Voided urine cytology is the standard noninvasive method for diagnosis in the detection of bladder carcinoma. Cytology is used to assess morphologic changes in intact cells. Unfortunately, however, the sensitivity of cytology is low, with various studies reporting values between 11% and 76%.5 Sensitivity depends largely on the degree of tumor differentiation. High-grade tumors with marked pleomorphism and distinctly abnormal nuclear features are identified more accurately. Small and/or well-differentiated tumors are less likely to exfoliate cells because intercellular attachments are better preserved and the degree of morphological departure from normal is smaller, complicating cytologic recognition.6This results in poor sensitivity in low-grade and early-stage cancers. Several other factors affect the sensitivity of cytology, including specimen quality, number of exfoliated cells, and pathologist expertise. The overall low sensitivity of cytology is due to its low sensitivity in detecting low-grade bladder tumors.7 In addition, instrumentation may cause reactive cellular changes, contributing to variability in interpretation. Falsepositive reports of malignant cells are uncommon, but ambiguous reports of atypical cells are frequent. Bladder wash cytology yields more tumor cells in the sample and is more sensitive in identifying cancer, especially for high-grade tumors, but it also yields a higher false-positive rate than voided urine cytology.8 Noninvasive urine markers can offer an alternative to the standard means of detecting bladder cancer or can be used as an adjunct to cystoscopy.9 Genetic aberrations The study of genetic aberrations commonly associated with urothelial carcinoma provides a more objective assessment for diagnosing and detecting recurrent disease. Homozygous loss of chromosome band 9p21, the site for the tumor suppressor gene p16, is a known early genetic event in the development of papillary carcinoma and urothelial CIS.10 Increased chromosomal instability and aneuploidy have been implicated in tumor progression. A study by Sokolova et al of 9 genetic markers for detecting urothelial carcinoma showed that polysomy of chromosomes 3, 7, and 17 and deletion of 9p21 were the most sensitive and specific markers, detecting 95% of recurrent urothelial carcinoma. 11 Halling et al established that a threshold of 5 or more cells with polysomy was 84% sensitive and 92% specific for detecting recurrent urothelial cancer.10 Fluorescence in situ hybridization A commercial FISH assay (UroVysion), which includes probes for the centromeres for chromosomes 3, 7, and 17 and has a locus-specific probe for 9p21, was developed to screen for recurrent urothelial carcinoma and was recently approved by the US Food and Drug Administration (FDA) for diagnostic studies. Initial comparisons of urine cytology with FISH for detecting bladder cancer recurrence showed that FISH yielded a greater sensitivity.12 FISH is 42-83% sensitive for detecting pTa and pT1 lesions and 92-100% sensitive for pT2-4 invasive lesions in patients with known bladder cancer, while urine cytology yields sensitivities of 24-50% for pTa and pT1 lesions and 78-85% for pT2-4 invasive lesions.13 For suspected new cases of urothelial carcinoma, cytology yields a reported diagnostic sensitivity of 48%, while no data are available for FISH evaluation of these cases.14 Laudadio et al compared the diagnostic sensitivity of FISH with concurrent biopsy and cytological assessments.15FISH analysis was found to yield a high sensitivity for detecting new cases of urothelial carcinoma, as well as recurrences. Their study showed FISH detected 95% of cases with highgrade carcinoma, while cytology detected 41% of such cases. FISH yielded an overall specificity of 65%, compared to 93% with cytology. From this data, the authors concluded that FISH is considerably more sensitive and only slightly less specific than cytology in diagnosing urothelial carcinoma. They recommended FISH as a useful initial diagnostic tool in patients suspected of both new and recurrent bladder cancer. Nuclear matrix protein-22 Nuclear matrix, first described in 1974, is the nonchromatin structure that supports nuclear shape and organizes DNA. It also takes part in DNA replication and transcription, as well as RNA processing.16,17,18 NMP-22 is involved in the proper distribution of chromatin to daughter cells during cell division and is found in the nuclear matrix of all cell types. NMP-22 is thought to be released from the nuclei of tumor cells after they die and can be detected in the urine. Research has found that persons with bladder cancer may have urinary NMP-22 levels up to 25 times that in healthy persons.19 The NMP-22 BladderChek test is an in vitro immunoassay intended for the qualitative detection of NMP-22 in urine. It determines whether NMP-22 is present in urine and provides an absolute positive or negative test result, much in the same manner as a pregnancy test. The NMP-22 assay detects elevated amounts of nuclear mitotic apparatus protein, a component of the nuclear matrix essential for cell division that is released into the urine during cell death. Unlike cytologic examinations and FISH-based tests, detection of NMP-22 protein does not depend on the recovery of intact cells. It is a painless and noninvasive assay that provides results within 30 minutes and is the only in-office test approved by the FDA for the diagnosis of bladder cancer. Grossman et al compared the NMP-22 BladderChek test with cystoscopy and voided urine cytology for surveillance of recurrent bladder cancer.6 Initial cystoscopy alone detected 91% of the cancers. The combination of the NMP-22 test with cystoscopy increased overall sensitivity to 99% (P =0.005). The NMP-22 test was significantly more sensitive than cytologic analysis of voided urine. The authors concluded that, when combined with cystoscopy, the NMP-22 test improves the detection of recurrence in patients with a history of bladder cancer. Unlike cytologic analysis, this test does not require expert analysis or laboratory time, does not depend on intact cells, and provides unambiguous results. In addition, the NMP-22 test provides results during the patient visit, and its cost is less than half that of cytology. Of concern with the NMP-22 assay is its variability of performance in detecting bladder cancer. A report by Shariat et al assessed the variability in the diagnostic performance of NMP-22 for detecting recurrence and progression in patients with Ta, T1, and/or CIS TCC of the bladder.20 NMP-22 voided urine levels were measured in 2,871 patients who underwent office cystoscopy for monitoring previous stage Ta, T1, and/or CIS bladder cancer at 12 institutions. Their results showed that the manufacturer cutoff of 10 U/mL detected 57% of cases with a 19% false-positive rate. For each NMP-22 cutoff assessed, NMP-22 had a higher sensitivity for detecting grade III and stage T2 or greater bladder cancer than for detecting any cancer. No optimal cutoffs for detecting any or aggressive bladder cancer could be derived based on NMP-22 values. The authors concluded that there is a substantial degree of heterogeneity in the diagnostic performance of NMP-22 applied to populations from different institutions. There was no clearly defined NMP-22 cutoff, but there was a continuum of risk for recurrence and progression. Conclusions Several reviews have been performed to assess the myriad urine markers proposed for bladder cancer surveillance. They note that none of the markers has been proven sensitive and specific enough to replace cystoscopy.21 While FISH and NMP22 are promising, the clinical evidence is insufficient to warrant the substitution of the cystoscopic follow-up scheme with any of the currently available urine marker tests.22 If FISH and NMP-22 are considered to have some utility when used to complement or replace cytology, a dilemma arises when their results conflict with each other. Of particular interest is how to treat a patient with positive cytology and/or FISH findings when cystoscopy findings are negative. Because cytology is the most reliable urine test for detecting bladder cancer, a positive cytology finding should be treated as cancer until proven otherwise. If cystoscopy findings are negative in the setting of positive cytology findings, further evaluation of the urinary tract is required. The upper urinary tract should be evaluated with contrast imaging. Cystoscopy with bilateral retrograde pyelography and bilateral ureteral washings should be performed. At the time of this procedure, ureteroscopy may also be performed if possible upper tract disease is suspected. The urinary tract distal to the bladder— the shorter urethra in women or the longer urethra in men, with its prostatic, bulbar, and penile portions—must also be assessed during cystoscopy. If the findings of all of these examinations remain negative, one must maintain a heightened suspicion and perform routine surveillance with more regularity. In the setting of negative cystoscopy findings, negative urine cytology findings, and positive FISH findings, 2 possible scenarios arise. This result is either falsely positive, or it may be an anticipatory positive result, meaning that such patients have a 30% chance of developing a bladder tumor over 2 years, despite having negative cytology and cystoscopic evaluation findings. Patients in this category should also undergo surveillance with increased frequency (see Table 2). Table 2. Clinical Findings and Recommended Action Cysto Urine FISH Action scopy Cyto Findings Findings logy Findings Negative Negative Negative Routine follow-up Negative Negative Positive Increased frequency of surveillance, whether FISH findings are false-positive or anticipatory positive Negative Positive Negative Cancer until proven otherwise or Upper tract imaging positive with contrast Cystoscopy with retrograde pyelography, washings, and/or ureteroscopy Evaluate urethra Increased frequency of surveillance upon negative findings Patients who have undergone radical cystectomy require routine surveillance to monitor for local recurrence or the development of metastatic disease. Abdominal and pelvic CT scanning and chest radiography should be performed annually. Some patients with more adverse pathology at the time of cystectomy (eg, locally advanced disease, lymph node metastases) may require more frequent imaging. The retained male urethra is at risk for cancer recurrence after radical cystoprostatectomy. Urethral recurrence occurs in approximately 7% of patients after cystoprostatectomy. 1. Cancer involving the prostate (urothelium or stroma) at the time of cystoprostatectomy is the most significant risk factor for urethral recurrence. 2. Monitoring the retained urethra has historically included periodic urethral cytology with subsequent biopsy, if indicated. However, some small studies have suggested monitoring with urethral washings does not confer a survival benefit.23 3. Gross hematuria or bloody urethral discharge requires immediate workup. 4. A positive urethral cytology or biopsy finding warrants immediate urethrectomy. Complications 1. The morbidity of untreated bladder cancer is significant and includes hematuria, dysuria, irritative urinary symptoms, urinary retention, incontinence, ureteral obstruction, and pelvic pain. 2. The radical cystectomy perioperative mortality rate is 12%. 3. The local recurrence rate is 5-10%; however, it increases to 15-25% for T3-T4 disease. 4. The 2 most common complications are small-bowel obstruction and ureteroenteric stricture (see Table 3 at the end of this section). 5. Radical cystectomy The reported overall early and late complication rate associated with radical cystectomy is approximately 25%-30%. However, this may be an underestimation of the true complication rate because of a lack of standardized reporting in published studies. Many patients undergo a radical cystectomy and have multiple comorbid health risk factors (eg, advanced age, cardiovascular disease, pulmonary disease). Despite these difficulties, this procedure may be performed safely in patients older than 80 years. Following a radical cystectomy, all men are impotent if the parasympathetic nerves from the pelvic plexus (S2S4) to the corpora cavernosum are not spared at the time of surgery; however, a nerve-sparing approach may reduce the impotency rate to approximately 40-50%. 6. Orthotopic neobladder With the recent advances in surgical technique, this procedure is becoming the diversion of choice. Risk factors include daytime and nighttime urinary incontinence of approximately 10% and 15%, respectively. Urinary incontinence may develop from multiple factors, including injury to the external urethral sphincter, increased urine production from solute absorption, and relaxation of the external sphincter, which is greater at night. Table 3. Most Common Complications of Radical Cystectomy Early Complications Rate,% Late Complication Rate,% Ileus 5.9 Small bowel 7.4 obstruction Wound infection 5.5 Ureteroenteric 7.0 stricture Sepsis 4.9 Renal calculi 3.9 Pelvic abscess 4.7 Acute 3.1 pyelonephritis Hemorrhage 3.4 Parastomal hernia 2.8 Wound dehiscence 3.3 Stomal stenosis 2.8 Bowel obstruction 3.0 Incisional hernia 2.2 Enterocutaneous 2.2 Fistula 1.3 fistula Rectal injury 2.2 Rectal <1 complications Prognosis 1. Non–muscle invasive bladder cancer has a good prognosis, with 5-year survival rates of 82-100%. The 5-year survival rate decreases with increasing stage, as follows: a) Ta, T1, CIS – 82-100% b) T2 – 63-83% c) T3a – 67-71% d) T3b – 17-57% e) T4 – 0-22% 2. 3. 4. 5. 6. Prognosis for metastatic urothelial cancer is dismal, with only 5% of patients living 2 years after diagnosis. Early diagnosis and improvements in treatment of bladder cancer may be responsible for the improved survival rate. Further studies of molecular determinants of bladder cancer development and progression aid in prevention, earlier diagnosis, and treatment. Much progress has been made in the treatment of advanced bladder cancer; however, researchers must further elucidate optimal agents and regimens. The underlying genetic changes that result in a bladder tumor occur in the entire urothelium, making the whole lining of the urinary system susceptible to tumor recurrence (ie, 70% within 5 y). Non–muscle invasive bladder cancer The risk of progression, defined as an increased tumor grade or stage, depends primarily on the tumor grade. The risk of progression increases with tumor grade, as follows: a) Grade I – 10-15% b) Grade II – 14-37% c) Grade III – 33-64% CIS alone, or in association with Ta or T1 papillary tumor, carries a poorer prognosis and a recurrence rate of 6392%. Diffuse CIS is an especially ominous finding, with 78% progressing to muscle-invasive disease in one study. Other risk factors for recurrence and progression include the tumor size, multifocality, number of tumors, high tumor grade, advanced stage, the presence of CIS, and the time interval to recurrence. Patients with tumor recurrences within 2 years, and especially with recurrences within 3 months, have an aggressive tumor and an increased risk of disease progression.