ANALYTICAL PROCEDURES. Metals in water Metals were
advertisement

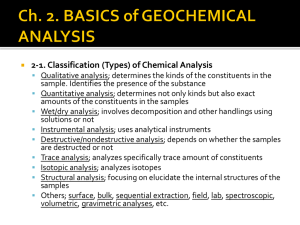
ANALYTICAL PROCEDURES. Metals in water Metals were extracted from seawater using a bulk-extraction technique with Analytical Grade Chelex 100 chelating resin (Bio-Rad Laboratories).The resin was preconditioned by weighing 5.0 g Chelex-100 (200–400 mesh) and soaking it in 15 mL of 2.5 M HNO 3 for 4 hours. The sample was placed in a separation column with 20 mL of 2.5 M HNO3, washed with 10 mL deionized water, eluted with 15 mL of 2 M NH 4OH, and rewashed with 15 mL of deionized water. The pH of the seawater samples was adjusted to 5.0–5.5 with concentrated NH4OH, and cations separated by eluting with 1.0 M NH4OH. The column was washed again with 10 mL of deionized water and metals were eluted with 10 mL of 2.5 M HNO3. Samples were analyzed for Al, Ba, Ni, Cd, Pb, Zn, V, Cr, Cu, Fe, and As by hydride generation (Arbab-Zavar and Howard, 1980) using inductively coupled plasma–atomic emission spectrometry (ICP-AES; Thermo Jarrell Ash; model Atom Scan 16). After every set of 10 samples, the ICP-AES was recalibrated using a multi-elemental standard solution (SPEX CertiPrep Inc., and Perkin-Elmer Corporation PE-Pure standard), both traceable to National Institute of Standards and Technology via the standard curve approach. The validity of the analytical procedure was assessed by accuracy and precision tests to compare the measured and certified reference values of the multi-element spiking solution for water and soil samples (ICPSSWS of High-Purity Standards, Charleston, SC, USA, traceable to National Institute of Standards and Technology). The absolute recoveries of the reference standard were between 85 % and 90 %. 2.5.2 Metals in sediments The sediments were oven dried at 60 oC for 4 hours and homogenized. A subsample (0.2 to 1.0 g) was lixiviated in a microwave digestion system (CEM Mar 5) with HNO3: HCl (3:9 v: v) following USEPA method 3051 (USEPA, 1997). The extracted solutions diluted to 50 ml with Milli-Q deionized water, and the contents of the flasks were transferred to 125 mL high-density polyethylene sample bottles for storage. Metal concentrations in uncontaminated seawater, field blanks, and laboratory blanks were always at or below the respective method detection limits (MDL), and the validity of the analytical procedure was assessed as described before. Trace metal analysis was carried out by ICP-AES following USEPA method 6010C (USEPA, 2007a). The sediment samples were processed in batches, each with a set of QC samples that included a procedural blank, laboratory control sample, matrix spike sample, and sample duplicate. 2.5.3 Petroleum hydrocarbons in water and sediments Samples were analyzed for total petroleum hydrocarbons (TPH), resolved plus unresolved hydrocarbons including normal alkanes from n-C8 through n-C40, using high-resolution gas chromatography/flame ionization detector (GC/FID) with an Agilent Technologies instrument. Water samples were extracted three times into 30 ml of spectrophotometer grade carbon tetrachloride, using fresh solvent, and all the solvent combined in a volumetric flask. The combined extracts were filtered through glass fiber wool, transferred to acid-soaked, pre-cleaned amber glass flasks, and stored at 4 °C until analysis. All samples spiked with a surrogate internal standard, after solvent extraction, were concentrated by evaporation (Kuderna-Danish) at 80 °C. The surrogate compound for TPH/alkanes was o-terphenyl. The samples were spiked with 20 µl of 5-α-androstane as recovery internal standard (RIS). TPH and selected alkanes in the n-C8 to n-C40 hydrocarbon were analyzed following the guidelines provided in EPA-8015 method (USEPA, 2007b). This instrument was calibrated with a six-point calibration of target compounds to demonstrate the linear range of the analysis. The water and sediment samples were processed in four batches, each with a set of quality control (QC) samples that included a procedural blank, laboratory control sample, matrix spike sample, and sample duplicate. The absolute recovery of the reference standard ranged between 44 to 118%. Approximately 30 g. of homogenized sediment were spiked with SIS and serially extracted three times with methylene chloride using an accelerated solvent extractor. The combined extracts were dried over sodium sulfate and concentrated to 1 mL of solvent using a nitrogen evaporator (Kuderna-Danish). The concentrated extracts were treated with activated copper to remove sulfur, and the extract weights were determined gravimetrically. One-half of the extract was reserved; the other half was processed through an alumina gravity column to isolate the hydrocarbon fractions of interest. 2.5.4 Polycyclic aromatics hydrocarbons in water and sediments Well-established alkyl homologue pattern recognition and integration techniques were used to determine concentrations of PAHs. Samples were extracted with methylene chloride, spiked with surrogate internal standards, and concentrated using an evaporator (Kuderna-Danish) at 80 °C. Extracts were separated by highperformance liquid chromatograph (HPLC), equipped with a size exclusion column, using methoxychlor and perylene as standard. The surrogate compounds for PAHs and petroleum biomarkers were 5-β-colane. The volume was reduced to approximately 60 mL. The resulting extracts were dried with sodium sulfate, concentrated to 2 mL, and spiked using naphthalene-d8, phenanthrene-d10, and chrysene-d12 as RIS and later analyzed by gas chromatography mass spectrometry (GC/MS) with the MS operating in the SIM mode, following the general guidelines provided in EPA method 8270D (USEPA, 2007c). The molecular ion of the alkyl homolog was extracted, and the areas of the individual isomers summed by a straight line, using baseline integration. 2.5.5 Organism tissue analysis Samples were logged into the laboratory on arrival, and the temperatures of the coolers were recorded. The biota samples were analyzed for PAHs. Whole body fish were homogenized using a Tissumizer™ with titanium probes. Approximately 5 g of tissue from each sample was used for analysis. A separate 5 g aliquot was removed for dry weight determination (dried at 105 °C). Sample extraction was performed by accelerated solvent extraction (ASE) using methylene chloride as the extraction solvent. The tissue was transferred to a 50-mL beaker, spiked with SIS, and mixed with approximately 5 g of Hydromatrix™. The surrogate compound for PAHs was 5-β-colane. After allowing the samples to stand for approximately 30 minutes, they were transferred to the ASE extraction cells and extracted three times with methylene chloride. The extracts were dried over sodium sulfate and reduced to approximately 3 mL by nitrogen evaporation. The extracts were then processed through an HPLC, equipped with a size exclusion (type) column, and further refined through alumina columns. The final extract was spiked with acenaphthene-d10 and analyzed for the PAHs content by GC/MS. 2.6. Quality assurance/quality control In addition to the analysis and monitoring of the QC samples, the QA/QC measures associated with the program included documentation of GC and GC/MS instrument calibration and response factor stability, monitoring of interference and/or contamination, and documentation of analytical accuracy and precision. The Quality Assurance Unit monitored the analytical components of the project according to our standard operating procedures to ensure the accuracy, integrity, and completeness of the data. Analytical project staff members were responsible for ensuring that sample tracking, sample preparation, and analytical instrument operation all met the quality control criteria. For a valid initial calibration, a percent relative standard deviation for each response factor (RF) of < 25 percentage was required for GC/MS and < 15 % for GC-FID. The calibration was verified prior to sample analyses every 10 samples or every 12 hours, whichever was more frequent. The RF for each analyte was required to be within 25 % of the initial calibration RF. Procedural blanks were considered acceptable if analytes were not detected at concentrations five times greater than the MDL. Target criteria for the recovery of surrogate and matrix spike compounds were 40–120 %.Results of the standard reference material analyses were expected to be within 30 % of the certified value plus the 95 % confidence level of the analyte concentration when it was five times greater than the sample specific MDL . References Arbab-Zavar MH, Howard AG (1980) Automated procedure for the determination of soluble arsenic using hydride generation atomic-absorption spectroscopy. Analyst, 105: 744-750. USEPA. United States Environmental Protection Agency. (1997). Method 3051-A.Microwave assisted acid dissolution of sediments, sludges, soils and oils.2n edition, United States Environmental Protection Agency, Washington, D.C. USEPA. United States Environmental Protection Agency. (2007a). Method 6010C. Determination of Inorganic Analytes by Inductively Coupled Plasma-Atomic Emission Spectrometry”, Test Methods for Evaluating Solid Waste, SW-846. United States Environmental Protection Agency, Washington, DC, December USEPA. United States Environmental Protection Agency. (2007b). Method 8015C. Nonhalogenated organics by gas chromatography. Revision 3, United States Environmental Protection Agency, Washington, DC February USEPA. United States Environmental Protection Agency. (2007c). Method 8270D. Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS).Revision 4, United States Environmental Protection Agency, Washington, DC ,February.