Ch. 4.1-4.2 Packet – 2015-16
advertisement

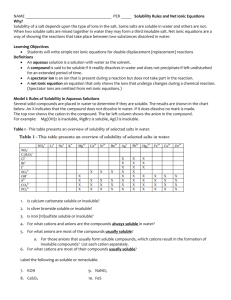
Honors Chemistry Name: __________________________________________ Date: _____________ Mods: _________ Ch. 4.1: Properties of Aqueous Solutions 1) Solution - homogeneous mixture of two or more pure substances (solute(s) dissolved in solvent) Solvent – substance present in the _______________ quantity Solute – all other substances present in ________________ quantities 2) Water: the Universal Solvent How are substances dissolved in water? o Water is known as the “universal solvent” due to the fact that more substances dissolve in water than any other chemical (it has to do with the high polarity of each H2O molecule) o o Polarity – physical property of a substance which results from an ___________ distribution of positive and negative charge in a molecule or chemical bond However, not all substances can dissolve in water and those that do dissolve in water dissolve to varying degrees: ______________ – a substance which dissolves easily and completely in water, forming an aqueous solution ______________ (or partially) Soluble – a substance which only dissolves a tiny bit in water leaving the majority or the substance behind in its original state of matter _________________ – a substance that does not dissolve in water and remains in its original state of matter separate from the H2O molecules (no interaction) The process of solvation (sometimes known as dissociation): During this solvation process, the cations and anions that make up a soluble ionic solute become ___________________ (or separated) from one another; these ions are then attracted to and surrounded by the water (solvent) molecules These dissociated ions are free to ___________ throughout the solution; these moving ions can then carry a current throughout the solution resulting in electrical _____________________ (movement of charged particles through a medium) 3) Determining Solubility of Compounds in Water: To know if an ionic compound is soluble, you must consult the solubility rules o PRACTICE: Are the following ionic compounds soluble or insoluble in water? Be(C2H3O2)2 MgS _______________ _______________ BaSO4 _______________ K3PO4 _______________ In order to determine if covalent compounds are soluble in water, you must determine the molecular polarity of the compound (this is something you have not yet learned how to do) Acids are not explicitly listed in the solubility rules because all acids that you will deal with are aqueous solutions (and thus soluble) o Acid – substance that produces ______ ions when dissolved in water (Arrhenius) The only types of bases that you will see at this point will be handled like ionic compounds o Base – substance that produces ______ ions when dissolved in water (Arrhenius) 4) Strong vs. Weak Acids & Bases: In the picture below, which acid is weak and which is strong? What is different about the contents in each of these beakers? Strong Acids & Bases: o These dissociate ___________________ and exist as ________% ions in solution o There are only 7 strong acids that exist and they must be MEMORIZED: o Sulfuric (H2SO4) Chloric (HClO3) Perchloric (HClO4) The strong bases are soluble ionic hydroxide compounds (see solubility rules – excluding ammonium hydroxide) Hydrochloric (HCl) Hydrobromic (HBr) Hydroiodic (HI) Nitric (HNO3) Alkali metal hydroxides (LiOH, NaOH, KOH, etc.) Calcium hydroxide, Ca(OH)2 Strontium hydroxide, Sr(OH)2 Barium hydroxide, Ba(OH)2 Weak Acids & Bases: o These only _______________dissociate in solution (only a ________ ions exist) o For acids and bases: if it’s not strong, then it’s weak 5) Predicting Ions in Solution: If a substance is _________________, then you can predict what types of and how many ions are present in the solution when each compound dissociates o PRACTICE: What ions are present in the solutions below? How many of each ion is present as the compounds below dissociate? Ammonium Sulfate: _____________________________________________________ Phosphoric Acid: _______________________________________________________ 6) Electrolytes An electrolyte is a substance which possess all of the following __________ qualities: o is soluble in water o dissociates into ions when dissolved o AND conducts electricity in its aqueous form Aqueous solutions can be classified into three categories based on how electrolytic they are: o o ______________ Electrolytes Dissolves readily in water (soluble), dissociates almost completely into ions (~_______%), and is a good conductor of electricity INCLUDES: all ________________ ionic compounds, all ____________ acids & bases ______________ Electrolytes Dissolves in water to a degree (slightly soluble), dissociates only _________________ into ions(solution exists mostly as molecules or crystals surrounded by water = very, very few ions present in solution), and is a poor conductor of electricity INCLUDES: _____________ soluble ionic compounds, ____________ acids & bases (ex: acetic acid, ______________ which dissociates into only ~ ______% ions) o ________electrolytes Does _________ dissociate into any ions (dissolved substances consist of _________ molecules surrounded by H2O) and does NOT conduct electricity INCLUDES: all ________________ ionic compounds (they can’t even form solutions) and most ________________ compounds (with the exception of covalent acids/bases) How to Classify Electrolytic Solutions: o o First determine if the substance is ionic, covalent, or acid/base… If _________________, determine if it is soluble… (see solubility rules) If soluble = strong electrolyte If insoluble = nonelectrolyte If __________________ and not as acid/base… These do not contain any ions and are therefore considered nonelectrolytes Examples include sugars & organic hydrocarbons If _________________, determine if it is a strong acid (must have memorized)… If strong acid = strong electrolyte If weak acid = weak electrolyte PRACTICE: Classify the following compounds based on their ability to produce solutions which are strong electrolytes, weak electrolytes, or nonelectrolytes. compound 1 2 3 4 5 6 CaCl2 HNO3 Ag3PO4 HCHO2 KOH C2H5OH ionic, covalent, acid, or base soluble or insoluble strong, weak, or nonelectrolyte Ch. 4.1: Solubility & Electrolytes WS Directions: Fill in the table below to classify the given compounds as ionic, determine their solubility, and classify them as strong, weak, or nonelectrolytes. If the compound is covalent, write “unknown” in the solubility column because, at this given time, you will be unable to determine whether or not a covalent compound is soluble (as it is based on molecular polarity). compound 1 NiBr2 2 Cs2S 3 H3PO4 4 (NH4) 2SO4 5 C5H10O 6 SrCO3 7 Ca(C2H3O2)2 8 PbCl2 9 Cu(OH)2 10 K2CO3 11 AgNO3 12 Mg(OH)2 13 HClO4 14 C4H9OH 15 CuCO3 16 HC3H5O2 ionic, covalent, acid, or base 17. Based on the picture shown at right, identify the following solutions(A, B, and C) as: Strong Electrolyte: ________ Weak Electrolyte ________ Nonelectrolyte: ________ soluble or insoluble strong, weak, or nonelectrolyte Ch. 4.2: Precipitation Reactions (aka: Double Replacement Reactions) Precipitate (ppt.) – an _________________ solid formed from the double replacement reaction of two aqueous solutions --------------------------------------------------------------------------------------------------------------------------------------Directions: Predict the products formed by the combination of the following solutions. If a precipitate forms, write a balanced chemical equation for the reaction and underline the precipitate formed. If all products are aqueous, just write “NR” (no reaction). 1. ____Li2CO3 (aq) + ____AgNO3 (aq) 2. ____NaOH (aq) + ____K2SO4 (aq) 3. ____BaCl2 (aq) + ____H3PO4 (aq) 4. ____FeSO4 (aq) + ____Pb(NO3)2 (aq) 5. ____K2SO4 (aq) + ____FeCl3 (aq) 6. ____Mg(C2H3O2)2 (aq) + ____(NH4)2S (aq) 7. Potassium sulfide and zinc chloride are combined. 8. Cesium phosphate and cadmium (II) acetate are combined. 9. Ammonium iodide and strontium hydroxide are combined. Ch. 4.2: Complete Ionic Equations, Net Ionic Equations, & Spectator Ions Complete Ionic Equation (CIE) – rewrite a balanced chemical equation as the CIE to show which compounds dissociate into ________ (this is true only of soluble, strong electrolytes in the _____ state of matter) Spectator Ions – ions present on ___________ the reactant and product side of a CIE which play ______ ________________ __________ in the chemical reaction Net Ionic Equation (NIE) – after the CIE is written, cancel out all the _______________ ions so that the NIE includes only ions and compounds _____________ involved in the chemical reaction --------------------------------------------------------------------------------------------------------------------------------------Directions: Predict the products formed by the combination of the following solutions. Write the balanced complete ionic equations and net ionic equations for the precipitation reactions that occur when each of the following solutions are mixed. If no reaction occurs write “NR”. At the end, list all the spectator ions present in the reactions. 1. ____(NH4) 3PO4 (aq) + ____CaCl2 (aq) 2. ____Cr(OH)3 (s) + ____HNO3 (aq) 3. ____Pb(NO3) 2 (aq) + ____Li2S (aq) 4. ____CoBr3 (aq) + ____K2CO3 (aq) 5. Cesium carbonate reacts with sulfuric acid to form cesium sulfate, gaseous carbon dioxide, and water. 6. Separate samples of a solution of an unknown ionic compound are treated with solutions of AgNO3, Pb(NO3) 2, and BaCl2. Precipitates form in all three cases. Which of the following anions could be the anion of the unknown ionic compound: Br –, C2H3O2–, or CO32– ? (HINT: you must figure out which if these anions forms a precipitate with all three solutions) 7. Separate samples of a solution of an unknown ionic salt are treated with dilute solutions of HBr, H2SO4, and NaOH. A precipitate forms only with H2SO4. Which of the following cations could the unknown solution contain: Pb2+, Ba2+, or K+ ? (HINT: you must figure out which if these cations forms a precipitate with only the sulfuric acid and not the other two solutions)