Supporting Information Preparation of free
advertisement

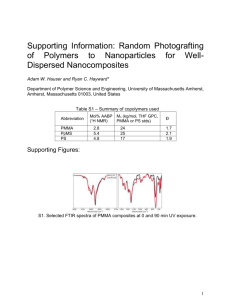
Supporting Information Preparation of free-standing two-dimensional colloidal crystal arrays Fei Xue, Zihui Meng*, Fenglian Qi, Min Xue*,Lili, Qiu School of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing, China, 100081 Experimental Materials Methyl methacrylate (MMA) was purchased from Sigma and used as received. Potassium persulfatewere obtained from Aladdin Co. Ltd. Toluene, DMSO, acetone, chloroform, ethyl acetate and acetonitrile were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water (Aquapro) was used for the experiment. Common glass slides (22×22×0.15 mm) and metal meshes (Weiss Experiment Products Co. Ltd.) were used in this research. Before use, the glass slides were immersed in a H2SO4-H2O2 mixture (7:3) for 12 h, and then rinsed with deionized water in an ultrasonic bath for three times and then dried for use. The metal meshes were used as received. 1 Preparation of monodisperse PMMA particles with different diameters Monodisperse PMMA particles of 262 nm diameter were polymerized by a previously reported emulsifier-free emulsion polymerization method1. These seed PMMA particles were washed thoroughly with deionized water and diluted with water to give a final concentration of 0.03 g/mL. PMMA particles with larger sizes of 530, 620 and 740 nm were prepared by a seeded emulsifier-free emulsion polymerization method. Briefly, 25 mL of the seed PMMA suspension solutions were added to 90 mL deionised water in a four-neck round-bottom flask with a condenser. The solution was deoxygenated by bubbling with nitrogen for half an hour. The solution was then heated to 80 ºC followed by injections of a 5 mL potassium peroxydisulfate solution (0.2 g) and a certain amount of MMA (5, 10, and 20 mL) was added through a side neck of the flask. A temperature sensor monitored the reaction temperature. The mixture was then refluxed for 45 min while being stirred at 300 rpm. After polymerization, the monodisperse PMMA particles of 530, 620, and 748 nm in diameter were washed with deionized water. The pure PMMA suspension was dried in drier under 60 ℃. 2 Preparation of free-standing 2D PMMA colloidal crystal arrays 20 mg dried PMMA particles of 530, 620 and 740 nm was added into 0.5 mL toluene and mixed for 30 s. During this time, PMMA particles partially dissolved in the toluene phase. Directly drop one droplet of the toluene suspension of PMMA particles on water surface where the particles spread to assemble into a close-packed 2D colloidal crystal array at the air/water interface in a 7.5 cm diameter glass dish. Free-standing 2D colloidal crystal array films formed upon toluene evaporation in air. Transfer these films by picking up them by glass slide and metal mesh. 3 Characterization of free-standing 2D PMMA colloidal crystal arrays Gold layers were sputtered onto the resultant 2D colloidal crystal arrays. The particles, their orderings and morphologies were examined with an SEM (Quanta FEG 250, FEI). Debye diffraction rings were measured to characterize the forward light diffraction of the 2D colloidal crystal arrays2. A 405 nm violet-blue laser pointer illuminated the surface of the 2D colloidal crystal arrays at normal incidence. The photographs of the CCHs were taken using a digital camera at an angle of ~30° between the light source/camera and the colloidal crystal array normal. The 2D colloidal crystal arrays were placed on an aluminum mirror. Fig. S1 Free-standing 2D PMMA colloidal crystal arrays prepare with 2 (a) and 4 (b) min operation time using toluene as solvent Fig. S2 Free-standing PMMA films prepared with different operation time (1、2、4、 6、9、12 min) using toluene/ethyl ether as solvent Fig. S3 SEM images of monodisperse PMMA particles with diameters of 530 (a), 620 (b), and 740 nm (c). The scale bars are 1μm in length. References 1. F. Xue, Z. Meng, Y. Wang, S. Huang, Q. Wang, W. Lu and M. Xue, Analytical Methods, 2014, 6, 831-837. 2. J.-T. Zhang, X. Chao, X. Liu and S. A. Asher, Chemical Communications, 2013, 49, 6337-6339.