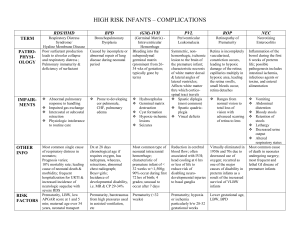
neo review febrero 2013
advertisement

NEO REVIEW FEBRERO 2013 Pain and Sedation in the NICU 1. Dennis E. Mayock, MD, and 2. Christine A. Gleason, MD + Author Affiliations 1. Division of Neonatology, Department of Pediatrics, University of Washington School of Medicine, Seattle, WA. Next Section Abstract Recognition and treatment of procedural pain and discomfort in the neonate remain a challenge. Procedural sedation and control of pain and discomfort are frequently managed together, often by using the same intervention. Therefore, although this article focuses on sedation, separating sedation from pain control is not always possible or wise. Despite significant progress in the understanding of human neurodevelopment, pharmacology, and more careful attention to how we care for sick infants, we still have much to learn. Protecting and comforting our fragile patients requires us to use poorly validated tools to assess and intervene to minimize distress, often applying data derived from adult patients to infants. Our first priority should be to minimize pain and distress. Further exploration of nonpharmacologic methods of procedural pain and distress control are needed. When pharmacologic intervention is necessary for procedural pain control and sedation, we need to use the least amount of drug that controls the pain and distress for the shortest period of time. As newer techniques and medications are introduced to clinical practice, we must demonstrate that such additions achieve their goal of sedation or pain control, and are safe over the lifetimes of our patients. Clinicians should identify appropriately the need for and use of sedatives and analgesics in the neonate. Previous SectionNext Section Practice Gaps 1. Many neonates do not receive adequate sedation for procedures in the NICU. 2. There is no evidence to guide appropriate sedation during extracorporeal life support. 3. The risks of some sedatives may outweigh their potential benefits. 4. Nonpharmacologic methods of sedation need further evaluation. Previous SectionNext Section Objectives After completing this article, readers should be able to: 1. Understand the challenges of using “pain scores” to assess the need for, and response to, neonatal sedation. 2. Describe the uses of sedation for facilitation of neonatal procedures. 3. Discuss pharmacologic approaches to neonatal sedation. 4. Discuss nonpharmacologic approaches to neonatal comfort care. Previous SectionNext Section Introduction Advances in neonatology have significantly improved morbidity and mortality, but pain, discomfort, and stress remain sad realities for infants in the NICU. Assessing, managing, and trying to limit these clinical realities while providing optimal care for critically ill neonates is challenging and increasingly controversial. Fortunately, there has been considerable research and much clinical dialogue aimed at developing best clinical practices in this problematic area. Sedation can be defined as the reduction of irritability or agitation, usually by administration of sedative drugs. In the NICU, sedation is used both to facilitate care (by limiting movement/agitation) and to minimize pain, discomfort, and stress during procedures and intensive care. Appropriate identification of the need for and use of sedatives and analgesics, based on the best available evidence, should be clinicians' objective. Previous SectionNext Section Assessment of Neonatal Sedation: “Pain Scores” More than 40 infant pain scales have been developed, most often for use in clinical trials to assess treatment efficacy; few have been validated for general clinical use for acute pain assessment. These pain scores are also frequently used to assess adequacy of sedation, even though they were not developed for this purpose nor were they designed or validated for assessment of chronic pain or discomfort associated with mechanical ventilatory support, or for use in paralyzed or neurologically compromised infants. The American Academy of Pediatrics (AAP) and the American Academy of Pediatric Dentistry have recently published an update on pediatric sedation, which recommends using a carefully staged process to plan for and carry out sedation for diagnostic and therapeutic procedures (Coté et al, 2006). Sedation can be categorized according to the level of consciousness, effect on protective airway reflexes, patency of the airway, and response to stimulation. The level of sedation can progress from conscious sedation to deep sedation to general anesthesia (Table 1). View this table: In this window In a new window Table 1. Levels of Sedation According to the American Academy of Pediatrics The optimal approach to sedation management should include reducing the frequency of painful procedures and environmental stressors, facilitating developmentally appropriate care, determining the best technique to minimize the pain and stress associated with procedures, delegating responsibility for pain/sedation assessment and treatment to the bedside nursing staff, and using a balanced multimodal approach to procedural sedation (Allegaert et al, 2009). Previous SectionNext Section Sedation for Mechanical Ventilation Use of mechanical ventilation in neonates who have respiratory failure is a common practice. In the past, sedation (most often with opiates) was frequently used in pediatric and adult ICU patients who required mechanical ventilation. Extrapolation of evidence from early studies in nonneonatal patients led to frequent use of opiate sedation in neonates during mechanical ventilation, with limited information as to safety and efficacy. However, in the past decade, use of pharmacologic sedation in the adult ICU has been significantly reduced because of concerns regarding adverse cognitive outcomes and longer duration of ventilator support. These concerns have led to a rethinking of this practice in both pediatric and neonatal ICUs. The following discussion is a historical review of this issue. Mechanical ventilation in neonates is associated with an increase in hormonal stress responses, including increased cortisol and catecholamine levels. In the past, infants who appeared uncomfortable while on ventilatory support demonstrated asynchronous respiratory effort (“fighting the ventilator”), compromised gas exchange, and altered stress responses. Clinical studies from the 1990s demonstrated that opiate treatment prevented these adverse effects and reversed the previously described hormonal stress changes. Opiate sedation decreased stress scores in ventilated newborns. In ventilated term infants, the severity of respiratory failure as assessed by using the oxygenation index directly correlated with the need for analgesia and sedation. More recently, with the introduction of surfactant replacement therapy and synchronized ventilatory technology, many of these previous problems with infants “fighting the ventilator” have been eliminated. Furthermore, clinical trials have shown that preemptive narcotic use in ventilated infants may actually have detrimental effects. A small randomized trial of routine morphine infusion in ventilated preterm infants concluded that morphine lacked a “measurable analgesic effect” and there was “absence of a beneficial effect on poor neurological outcome.” The larger clinical trial which followed (NEOPAIN, published in 2004) reported no beneficial effect of preemptive morphine infusions in ventilated preterm infants and an increased incidence of severe intraventricular hemorrhage in 27- to 29-week gestational age preterm infants. Indeed, additional bolus doses of morphine resulted in worse respiratory outcomes and longer requirement for ventilatory support. These controlled clinical trials provide no evidence that routine narcotic sedation during mechanical ventilatory support in neonates is beneficial. One approach to this dilemma has been to minimize the use of ventilatory support as much as possible. A recent Cochrane Reviews article evaluated the effects of preemptive opioid sedation on pain scales, duration of mechanical ventilation, mortality, growth, and development in neonates requiring mechanical ventilation (Bellù et al, 2008). The authors found no differences in mortality, duration of mechanical ventilation, or short- and long-term neurodevelopmental outcomes. However, very preterm infants given morphine took longer to achieve full enteral feeding. If morphine sedation prolongs time to full enteral feeds, we should expect an increase in the risk of complications related to the use of venous lines (bloodstream infections) and parenteral nutrition (cholestasis). Indeed, the duration of morphine use was a strong predictor for development of severe necrotizing enterocolitis. The overall conclusion of the Cochrane Reviews article regarding use of sedation during mechanical ventilation was that “there is insufficient evidence to recommend routine use of opioids in mechanically ventilated newborns.” Previous SectionNext Section Sedation for Procedures Infants undergoing intensive care endure many painful procedures, often several times each day. Although new pharmacologic and nonpharmacologic treatment strategies have been developed to decrease or eliminate some of this pain and stress, we have a long way to go in developing evidence-based “best practices.” Blood Sampling and Monitoring Heel sticks are routinely performed to obtain blood samples in neonates. The most appropriate method for relieving pain from a heel stick has yet to be determined. EMLA (eutectic mixture of local anesthetics; 2.5% lidocaine and 2.5% prilocaine) does not relieve the pain of a heel lance. Neonates experiencing venipuncture had lower pain scores than those who underwent heel stick. In neonates, venipuncture should be used preferentially over heel stick. The pain of arterial puncture can be decreased by infiltrating the site with 0.1 to 0.2 mL of 0.5% or 1% lidocaine using the smallest-gauge needle possible. Buffering the lidocaine with sodium bicarbonate is recommended to decrease the burning caused by lidocaine. EMLA may reduce the pain of arterial puncture. Endotracheal Intubation The use of premedication to minimize the pain and stress of endotracheal intubation benefits the neonate. However, concerns about rapid medication availability, ability to maintain the airway, and the ability to provide ongoing ventilatory support have caused controversy. Premedications typically include atropine, narcotics for sedation, and muscle relaxants. Atropine abolishes vagal bradycardia. Narcotics attenuate the increases in arterial blood pressure. Muscle relaxants attenuate the increases in intracranial pressure. Combination treatment decreases time and number of attempts needed to intubate the infant. When one is considering the use of medications for intubation, several questions need to be asked: Does the infant have adequate vascular access? What is the urgency of intubation need? Is the infant known to have a “difficult airway”? When was the last feeding? Can the infant be preoxygenated while avoiding gastric distension? If the decision is made to use medications for intubation, typical dosages include: Atropine 0.02 mg/kg fast intravenous (IV) push Fentanyl 2 μg/kg slow IV push (over at least 5 minutes) Vecuronium 0.1 mg/kg IV push Alternative muscle relaxants may include succinylcholine 1 to 2 mg/kg IV push or rocuronium 1 mg/kg IV push. Propofol has been used as a premedication for intubation. As a single agent, it is easier and faster to prepare compared with a preparation of three separate drugs. Propofol is a hypnotic agent without anesthetic properties. However, propofol is painful when injected into small veins, and extremely painful if it extravasates. A major advantage is continued spontaneous breathing during the intubation procedure. The dose is 2.5 mg/kg intravenously; this dose might need to be repeated. Concerns with the use of propofol for intubation in neonates include minimal published experience in neonates, uncertain pharmacokinetics and duration of action, restricted availability in some institutions, and incompatibility with peripherally inserted central catheter lines. Circumcision The 2012 AAP Technical Report on Male Circumcision recommends that analgesia be provided to infants undergoing circumcision. EMLA cream, dorsal penile nerve block, and subcutaneous ring block are all possible options. The AAP reports that subcutaneous ring block may provide the best analgesia and has published a videotape demonstrating the use of local anesthesia for this procedure. Subcutaneous ring block is more effective than EMLA or dorsal penile nerve block. Dorsal penile nerve block is more effective than EMLA. EMLA is superior to placebo for pain relief during circumcision. An effective method for applying EMLA in preparation for circumcision is to apply one third of the dose to the lower abdomen, extend the penis upward gently, pressing it against the abdomen, and then apply the remainder of the dose to an occlusive dressing placed over the penis. The dressing is taped to the abdomen so the cream surrounds the penis and is left on for 60 to 90 minutes. Another method is to apply the cream and then place plastic wrap around the penis in a tube-like fashion to help direct the urine stream out and away from the cream. Acetaminophen is ineffective for the management of acute pain associated with the circumcision procedure but may provide some analgesia in the postoperative period. Previous SectionNext Section Other Invasive Procedures Placement of a central venous catheter requires topical anesthesia with EMLA or infiltration of the skin with lidocaine. In addition, a parenteral opioid such as morphine or fentanyl is typically used. Consideration should also be given to regional blocks for central line placement if anesthesia expertise is available for this method. The pain of a lumbar puncture is compounded by both the needle puncture and the distress caused by the body position required for the procedure. EMLA has been shown to decrease the pain of lumbar puncture in children. Sedatives are generally not recommended. Chest tube insertion is painful and requires an intravenous opioid, adequate local analgesia (lidocaine), or both. Previous SectionNext Section Sedation for Imaging Procedures Imaging of neonates in the NICU typically includes routine diagnostic radiographs and ultrasound examinations, including echocardiograms. Sedation is rarely required for these procedures. More detailed imaging requires specialized scanning such as computed tomography or magnetic resonance imaging. Although they provide more specific diagnostic and predictive information, such imaging usually requires transportation of the infant from the NICU to distant facilities. For high-quality computed tomography and magnetic resonance imaging, sedation is often considered to minimize artifacts from patient movement. Sedation in these circumstances can add substantial risk and cost to the procedure. Various approaches to avoid the use of sedation have been employed. Sleep promotion is often used, with scanning scheduled soon after a feeding (30 minutes), use of immobilization (swaddling) and restraint devices, and administration of sucrose drops before and during the scanning. Use of pacifiers during magnetic resonance imaging scanning, while often calming to the infant, can add motion artifact. Previous SectionNext Section Sedation During Therapeutic Hypothermia Therapeutic hypothermia is used increasingly for neuroprotection in newborns who have neonatal encephalopathy. Shivering is an uncommon finding in neonates, but it has been described frequently in infants undergoing therapeutic hypothermia and is an adverse effect that counteracts the physiologic intent of this intervention. Morphine sedation is usually adequate to minimize shivering. Dexmedetomidine, clonidine, meperidine, and propofol have been used for this purpose in adults. Benzodiazepines should be avoided because they can mask seizure activity. Pharmacologic neuromuscular blocking agents are not used unless the patient has uncontrollable shivering or generalized clonus that cannot be controlled with other sedatives or anticonvulsants. Previous SectionNext Section Sedation During Extracorporeal Life Support Sedation and analgesia are frequently needed during extracorporeal life support (also known as extracorporeal membrane oxygenation [ECMO]) to prevent cannula displacement with body movement. Muscle relaxants are also often used. No controlled trials have been published to provide guidance. It is important to understand that multiple factors influence drug pharmacokinetics during ECMO and that prediction of appropriate dosing is not possible. Factors leading to need for increased drug doses: Increased volume of distribution from ECMO circuit priming volume Binding/sequestration of drugs in oxygenator/other circuit components Hemofiltration of small molecules Factors leading to need for decreased drug doses: Renal and hepatic injury decreasing drug clearance Immature organ function in neonates Active drug metabolites Sedatives such as midazolam and opiates such as fentanyl are commonly used. However, achieving the desired effect of these medications frequently results in substantial dose escalation, requiring prolonged periods of slow drug weaning to avoid abstinence symptoms. Dexmedetomidine may be useful as a short-term adjunct for ECMO sedation. Previous SectionNext Section Pharmacologic Sedative Interventions The expected severity of the discomfort, its etiology, available administration routes, and potential adverse effects should all be considered when selecting a sedative for a planned procedure. Once medication administration has begun, careful monitoring for these effects can decrease potential adverse events. A key component of effective sedation management is ongoing assessment during and after an intervention or procedure. It is important to be prepared to rescue the infant if needed from a deeper level of sedation than originally planned. Nonopioid Analgesics Nonsteroidal anti-inflammatory drugs Nonsteroidal anti-inflammatory drugs (eg, indomethacin, ibuprofen) inhibit prostaglandin synthesis by inhibiting the action of cyclooxygenase enzymes. These agents have many physiologic effects, including sleep cycle disruption, increased risk of pulmonary hypertension, cerebral blood flow alterations, decreased glomerular filtration rate, alteration in thermoregulatory control, and changes in platelet function. Moreover, development of the central nervous, cardiovascular, and renal systems are all dependent on prostaglandins. These drugs have been used frequently in the NICU for pharmacologic closure of a patent ductus arteriosus, and aside from effects on renal and perhaps mesenteric circulation (which are difficult to separate from the patent ductus arteriosus), no clear-cut adverse effects have been reported. However, there is no robust evidence that they have analgesic efficacy in infants aged less than 3 months, thus limiting their use in neonates. Acetaminophen Acetaminophen is the most widely administered analgesic in patients of all ages despite little evidence of efficacy in infants less than 3 months of age. Acetaminophen inhibits the activity of cyclooxygenase in the central nervous system, decreasing the production of prostaglandins and peripherally blocking pain impulse generation. Neonates are able to form the metabolite that results in hepatocellular damage; however, it is inappropriate to withhold acetaminophen in newborns because of concerns of liver toxicity. The immaturity of the newborn’s cytochrome P-450 system may actually decrease the potential for toxicity by reducing production of toxic metabolites. Current recommendations call for less frequent oral dosing (every 8 to 12 hours in preterm and term neonates) because of slower clearance times and for higher rectal dosing due to decreased absorption. Oral dosages for acetaminophen are 10 mg/kg per dose every 6 to 8 hours for preterm neonates and 12.5 mg/kg per dose every 4 to 6 hours for term infants. The maximum recommended daily dose is 75 mg/kg for infants, 60 mg/kg for term and preterm neonates greater than 32 to 34 weeks’ postconceptual age, and 40 mg/kg per day for preterm neonates 28 to 32 weeks’ postconceptual age. Rectally administered acetaminophen has a longer half-life, but absorption is highly variable because it is dependent on the individual infant and placement of the suppository. It should also be noted that the drug may not be uniformly distributed throughout the suppository and therefore should be divided lengthwise if a partial dose is desired. The analgesic effect of acetaminophen may be additive when the agent is administered with opioids; coadministration may enable a decrease in the opioid dose and in corresponding opioid adverse effects. However, demonstration of this potential benefit awaits further study. Opioid Analgesics Opioids are believed to be the most effective sedative and analgesic for control of moderate to severe pain in patients of all ages. There is a wide range of interpatient pharmacokinetic variability. Opioid dosing depends on the severity of the anticipated procedural pain as well as the age and clinical condition of the infant. Opioids should be used in infants younger than age 2 months in a monitored setting only. Some clinicians propose a more conservative recommendation, restricting use of opioids to monitored settings for any infant younger than age 6 months. Morphine Morphine remains the gold standard for procedural pain management in neonates despite lack of proven efficacy in many circumstances. Morphine is metabolized in the liver by uridine diphosphate glucuronyltransferase into two active metabolites: morphine-6-glucuronide (M6G), a potent opiate receptor agonist, and a second metabolite, morphine-3-glucuronide (M3G), a potent opiate receptor antagonist. Both metabolites and some unchanged morphine are excreted in the urine. The predominant metabolite in preterm and term neonates is M3G. Because of slow renal excretion, the metabolites can accumulate substantially over time. There is a potential for late respiratory depression due to a delayed release of morphine from less well-perfused tissues and the sedating properties of the M6G metabolite. Because the predominant metabolite of morphine in infants is M3G, a potent opiate receptor antagonist, one should consider using the lowest dose possible to achieve the needed sedation. As we escalate the morphine doses, we are also increasing the levels of M3G and potentially interfering with our goal of adequate sedation. Doses as low as 1 to 5 μg/kg per hour can provide adequate sedation/analgesia, minimizing the risk of accumulation of high M3G levels with that metabolite’s prolonged half-life. Clearance or elimination of morphine and other opioids is prolonged in infants because of the immaturity of the cytochrome P-450 system. The rate of elimination and clearance of morphine in infants aged 6 months and older approaches that of adults. Chronologic age seems to be a better indicator of opioid metabolism in infants than gestational age. Infants are at greater risk for opioid-associated respiratory depression because of their immature respiratory center responses to hypoxia and hypercarbia. Furthermore, there is an increase in unbound or free morphine and M6G available to reach the brain as a result of the reduced concentration of albumin and α1-acid glycoproteins. Dosing recommendations currently reflect the wide range of interpatient pharmacokinetic variability. Previously, a 0.03 mg/kg bolus of IV morphine was suggested as a starting dose in nonventilated infants (Acute Pain Management Guideline Panel, 1992) whereas 0.05 to 0.1 mg/kg of IV morphine was recommended as an appropriate starting dose in ventilated infants. Recently, much lower doses have been recommended (0.025–0.05 mg/kg as a bolus dose or 1–5 μg/kg per hour as a continuous infusion). Titration to the desired clinical effect is required in adjusting both the dose and the frequency of administration. Furthermore, it is important to continually assess need and responses. As we further explore the use of morphine for analgesia and sedation in neonates, it is becoming concerning that some of the risks may outweigh any potential benefits. Fentanyl Fentanyl is 80 to 100 times more potent than morphine and causes less histamine release, making it a more appropriate analgesic/anesthetic choice for infants who have hypovolemia, hemodynamic instability, or congenital heart disease. Another potential clinical advantage of fentanyl is its ability to reduce pulmonary vascular resistance, which can be of benefit for infants who have undergone cardiac surgery, have persistent pulmonary hypertension, or need ECMO. Bolus doses of fentanyl must be administered slowly over a minimum of 5 minutes to avoid chest wall rigidity, a serious adverse effect observed after rapid infusion. Chest wall rigidity, which can result in difficulty or inability to ventilate, can be treated with naloxone or a muscle relaxant such as pancuronium or vecuronium. Recommended bolus doses are 1 to 2 μg/kg by slow IV infusion. Continuous infusion dosing should start at low levels (1–2 μg/kg per hour) and titrate to effect. Fentanyl is highly lipophilic. It has a quick onset and relative short duration of action. Because of this short duration of action, fentanyl is typically used as a continuous infusion for sedation and postoperative pain control. In infants age 3 to 12 months, total body clearance of fentanyl is greater than that of older children, and the elimination half-life is longer owing to its increased volume of distribution. Fentanyl has a prolonged elimination half-life in infants who have increased abdominal pressure. Due to tachyphylaxis, continuous infusions of fentanyl are frequently increased to maintain constant levels of sedation and pain management. Infusion dosing can reach substantial levels requiring prolonged withdrawal. A rebound transient increase in plasma fentanyl levels is a phenomenon known to occur after discontinuation of therapy in neonates. It is a result of fentanyl’s accumulation in fatty tissues, which may prolong its effects after continued use. Therefore, caution must be exercised in the use of repeated doses or a continuous infusion. Oral Opioids Oral methadone can be used to wean infants from long-term opioid use, although there are limited data on its efficacy and pharmacokinetics in this population. The respiratory depressant effect of methadone is longer than its analgesic effect. Methadone is metabolized very slowly via hepatic N-methylation resulting in accumulation in the body, and its half-life is very long (16 to 25 hours) in neonates. Codeine has been prescribed at 0.5 to 1 mg/kg orally every 4 hours as needed. Scarce data are available to recommend use of codeine in neonates. Most pharmacies supply acetaminophen and codeine in a set formula consisting of acetaminophen 120 mg and codeine phosphate 12 mg per 5 mL with 7% ethanol. The dose prescribed is limited by both the appropriate dose of codeine and the safe dose of acetaminophen. This combination is not recommended in neonates. Oxycodone is not recommended in neonates because no data are available for dosing guidelines. The liquid form is not universally available. Benzodiazepines Benzodiazepines such as lorezapam and midzaolam are sedatives that activate γaminobutyric acid receptors and should not be used in place of an appropriate pain medication because this class of medication has no analgesic effect. Benzodiazepines can be administered to decrease irritability and agitation in infants and to provide sedation for procedures. In ventilated infants, benzodiazepines can help avoid hypoxia and hypercarbia from breathing out of “sync” with the ventilator, although, as noted previously, this is not as much of a problem today as it was in the past. When given as continuous infusions, dosing often escalates rapidly to maintain apparent sedation, resulting in the need for prolonged weaning. These medications have been associated with abnormal neurologic movements in both preterm and term infants. In rats, prenatal exposure to diazepam results in long-term functional deficits and atypical behaviors, and exposure of 7-day-old mice to diazepam induces widespread cortical and subcortical apoptosis. Midazolam potentiates pain behavior, sensitizes cutaneous reflexes, and has no sedative effect in newborn rats. Whether these data can be extrapolated to human infants is unknown, but clinicians have reason to be concerned and should use these drugs with caution in the NICU. Dexmedetomidine Dexmedetomidine is a potent relatively selective α2-adrenergic receptor agonist indicated for the short-term sedation of patients in ICU settings, especially those receiving mechanical ventilatory support. It is administered by either bolus doses for short procedural sedation (1–3 μg/kg) or by continuous IV infusion (0.25–0.6 μg/kg per hour). Because dexmedetomidine does not produce significant respiratory depression, it has been used for procedural interventions in spontaneously breathing infants. As neonatologists become more familiar with dexmedetomidine, its use may increase. However, short- and long-term safety and effectiveness need to be assessed in human infants because preliminary work in a neonatal rodent model suggests that it may alter brain development. Topical Anesthetics EMLA cream reduces the pain of circumcision. It must be applied 60 to 90 minutes before the procedure; longer application times provide deeper local anesthetic penetration but may lead to toxicity. There is a slight risk of methemoglobinemia with the use of EMLA cream in infants and patients who are G6PD-deficient. This rare occurrence (methemoglobinemia) occurs when hemoglobin is oxidized by exposure to prilocaine. EMLA should not be used in patients who have methemoglobinemia or infants younger than age 12 months who are also receiving methemoglobinemiainducing drugs, such as acetaminophen, sulfonamides, nitrates, phenytoin, and class I antiarrhythmic agents. A study of 30 preterm infants found that a single 0.5-g dose of EMLA applied for 1 hour did not lead to a measurable change in methemoglobin levels. A systematic review concluded that EMLA diminishes the pain during circumcision. Limited efficacy was noted with pain from venipuncture, arterial puncture, and percutaneous venous line placement. EMLA did not diminish pain from heel lancing. Oral sucrose or glucose may be as effective as EMLA for venipuncture. Previous SectionNext Section Nonpharmacologic Analgesic Interventions There are numerous nonpharmacologic interventions available for prevention and/or relief of neonatal procedural pain and stress, either as the sole method of pain control or in combination with pharmacologic interventions. Because pharmacologic analgesia and sedation have not been proven effective and may be harmful, these alternative methods of pain and stress relief need to be assessed for their efficacy and safety. As clearly stated by Golianu et al (2007), “These therapies may optimize the homeostatic mechanisms of the infant, thereby mitigating some of the adverse consequences of untreated pain, as well as facilitating healthy physiologic adaptions to stress.” However, widespread adoption of specific interventions is not consistent. Following are summaries of currently available information on selected interventions: Breastfeeding reduces the physiologic and behavioral responses to acute procedural pain and stress in neonates and has been recommended as the first line of treatment. Nonnutritive sucking using pacifiers reduces pain responses to heel prick, injections, venipuncture, and circumcision procedures. Infant massage decreases plasma cortisol and catecholamine levels in preterm infants during painful procedures. Maternal skin-to-skin contact (kangaroo care) is associated with greater physiologic stability and reduced responses to acute procedural pain. Kangaroo care can decrease pain scores after vitamin K injections. Maternal rocking has been shown to diminish neonatal distress. Multisensory stimulation (simultaneous gentle massage, soothing vocalizations, eye contact, smelling a perfume, and sucking on a pacifier) has been associated with analgesia and calming of the infants in several reports from one unit. Music therapy may reduce the behavioral and physiological responses to acute procedural pain. Oral sucrose reduces pain behavior in preterm and term infants. The mechanism of oral sucrose analgesia is not known but may be related to stimulation of endogenous opioid release. Of all these methods and techniques, oral sucrose has been the most widely studied and used. As more data regarding the limitations of pharmacologic treatment are published, consideration of nonpharmacologic interventions will likely become more important and commonplace. Previous SectionNext Section Long-term Consequences of Neonatal Opioid Exposure Experimental Animal Studies Perinatal and neonatal opioid exposure in experimental animals is associated with both short- and long-term adverse neurologic effects. These effects should make clinicians wonder whether the use of such medications, with questionable benefits, should continue. Perinatal narcotic exposure restricts brain growth, induces neuronal apoptosis, and alters behavioral pain responses later in life. One area of particular concern to clinicians is the developing cerebral circulation, which is extremely vulnerable to physiologic perturbations and drug effects. Cerebrovascular effects of drug exposure early in development may have lifelong consequences, including increased risk for stroke. The acute effects of exogenous narcotics, including morphine, on the developing cerebral circulation have been described in piglets and include modulation of prostaglandin-induced pial artery dilation during hypoxia, alteration in endothelin production, and increases in endothelin A receptor messenger RNA expression. Endogenous opioids are important regulators of cerebrovascular tone and angiogenesis. Exposure to morphine in fetal sheep and neonatal rats permanently alters cerebrovascular control mechanisms. Permanent neurobehavioral and neuropathologic changes have reportedly been found in a rodent model of neonatal stress and morphine exposure. These animal studies demonstrated short- and long-term effects of neonatal morphine exposure, which is not surprising because opioid receptor–mediated signaling likely plays a role in several aspects of early brain development. However, the clinical relevance of these animal studies regarding the long-term effects of neonatal opioids is difficult due to species differences in timing of brain development, the development of opiate receptors and major neurotransmitter systems, and the pharmacokinetics of administered opioids. Clinical Studies Clinical studies addressing the short- and long-term effects of prolonged opiate use in human neonates are limited. The few that exist are contradictory and confounded by illness severity. Reversible “encephalopathic” changes in neonates receiving long-term sedative and narcotic infusions have been described. One study demonstrated no adverse neurodevelopmental outcomes in a small group of newborns who received morphine for a median of 5 days. A second study presented 5-year neurodevelopmental outcomes in very low birthweight infants exposed to prolonged sedation and/or analgesia (defined as >7 days of sedative and/or opioid drugs). Exposed very low birthweight infants had more severe or moderate disability at 5 years (42%) compared with those not exposed (26%). Preterm infants (23–32 weeks’ gestation at birth) evaluated at 36 weeks’ postconceptual age in the NEOPAIN study demonstrated neurobehavioral abnormalities if exposed to morphine during ventilatory support. Previous SectionNext Section Summary Recognition and treatment of procedural pain and discomfort in the neonate remain a challenge. There is still much to learn about human neurodevelopment, pharmacology, and how to best care for sick infants. Because we try to protect and comfort our fragile patients, and because external regulatory forces have required us to document discomfort by using poorly validated tools and to intervene to minimize distress, we often apply what is known from data in adult patients to infants. Our care should minimize the risks of adverse effects of both drugs and pain/stress on neurodevelopment. Further exploration of nonpharmacologic methods of procedural pain and distress control is needed. As newer techniques and medications are introduced to clinical practice, we must demonstrate that such additions achieve their goal of sedation or pain control, and also establish their safety over the lifetime of our patients. Escalation of drug doses may, in fact, be adding to our problem. Better tools are needed to help us optimize the outcomes of infants. American Board of Pediatrics Neonatal–Perinatal Content Specifications For therapeutic drugs commonly used in the neonate (eg, opiates, methylxanthines, barbiturates), know indications for their use, clinical effects, pharmacokinetics, adverse effects, and toxicity. Previous SectionNext Section Footnotes Author Disclosure Drs Mayock and Gleason have disclosed no financial relationships relevant to this article. This commentary does contain a discussion of an unapproved/investigative use of a commercial product/device. Abbreviations: EMCO; extracorporeal membrane oxygenation EMLA; eutectic mixture of local anesthetics M3G; morphine-3-glucuronide M6G; morphine-6-glucuronide Copyright © 2013 by the American Academy of Pediatrics Previous Section Suggested Reading 1. 1. Allegaert K, 2. Veyckemans F, 3. Tibboel D . Clinical practice: analgesia in neonates. Eur J Pediatr. 2009;168(7):765–770 CrossRefMedlineWeb of Science 2. 1. 2. 3. 4. Anand KJ, Hall RW, Desai N, et al ; NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682 CrossRefMedlineWeb of Science 3. 1. 2. 3. 4. Anand KJS, Anderson BJ, Holford NHG, et al ; NEOPAIN Trial Investigators Group. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth. 2008;101(5):680–689 Abstract/FREE Full Text 4. 1. Bellù R, 2. de Waal KA, 3. Zanini R . Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008;(1):CD004212 Search Google Scholar 5. American Academy of Pediatrics; American Academy of Pediatric Dentistry; Coté CJ, Wilson S; Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118(6):2587–2602 Abstract/FREE Full Text 6. 1. 2. 3. 4. 5. Golianu B, Krane E, Seybold J, Almgren C, Anand KJ . Non-pharmacological techniques for pain management in neonates. Semin Perinatol. 2007;31(5):318–322 CrossRefMedlineWeb of Science 7. 1. Mayock DE, 2. Gleason CA . Neonatal pain and stress: assessment and management. In: Gleason CA, Devaskar SU, eds. Avery’s Diseases of the Newborn. 9th ed. Philadelphia, PA: Elsevier; 2012:429–444 Search Google Scholar 8. 1. Walden M, 2. Carrier CT . Sleeping beauties: the impact of sedation on neonatal development. J Obstet Gynecol Neonatal Nurs. 2003;32(3):393–401 CrossRefMedline Neonatal Thrombocytopenia 1. Karen S. Fernández, MD*, and 2. Pedro de Alarcón, MD† + Author Affiliations 1. * Assistant Professor of Pediatrics, University of Illinois College of Medicine at Peoria and Children’s Hospital of Illinois, Peoria, IL. 2. †William H. Albers Professor and Chair Department of Pediatrics, University of Illinois College of Medicine at Peoria and Children’s Hospital of Illinois, Peoria, IL. Next Section Abstract Thrombocytopenia is one of the most common hematologic problems in the neonate. It affects up to 30% of all patients admitted to the neonatal intensive care unit (NICU). The causes of thrombocytopenia in neonates are diverse and include immune, inherited, and acquired disorders. The evaluation of the neonate with thrombocytopenia may be challenging. Developing a diagnostic strategy to evaluate the neonate with thrombocytopenia is key for the practicing clinician. Here, we provide a practical approach to the evaluation of the neonate with thrombocytopenia and an overview of its most common etiologies. Previous SectionNext Section Educational Gaps 1. Knowing the differential diagnosis and most likely etiologies of thrombocytopenia in the neonate will lead to more appropriate diagnostic evaluations and treatments. 2. Thrombocytopenia may be a symptom of a variety of congenital or acquired conditions in the neonatal period and prompt further diagnostic evaluations. Previous SectionNext Section Objectives 1. Describe the differences between neonatal and adult thrombopoiesis. 2. Provide a differential diagnosis of neonatal thrombocytopenia. 3. Describe the clinical presentation and management of the most common forms of thrombocytopenia during the neonatal period. 4. Explain and contrast the pathophysiology of neonatal autoimmune thrombocytopenia and alloimmune thrombocytopenia. 5. Describe the most common inherited causes of thrombocytopenia in the newborn. 6. Discuss the approach to treatment for the neonate with thrombocytopenia according to the etiology. Previous SectionNext Section Definition and Incidence Thrombocytopenia is defined as a platelet count under 150,000/μL. However, healthy neonates tend to have platelet counts in the range of 100 to 150,000/μL. Thrombocytopenia occurs in less than 1% of all neonates, but is one of the most common hematological problems in neonates, affecting 25% to 30% of all admissions to the neonatal intensive care unit (NICU). The evaluation and management of thrombocytopenia is a challenge for the neonatologist and hematologist, because it can be caused by multiple disease processes. Therefore, a review of the differential diagnosis, the pathophysiology, and the management of the newborn with thrombocytopenia is important. Previous SectionNext Section Platelets Production in the Newborn Platelets are tiny cellular fragments produced by megakaryocytes. Platelet production, or thrombopoiesis, is a complex process that consists of four main steps: 1. Production of thrombopoietin (Tpo) as the thrombopoietic stimulus. 2. Generation and proliferation of megakaryocyte progenitors. 3. Maturation of megakaryocytes characterized by a progressive increase in nuclear ploidy (the number of sets of chromosomes in a given cell) and cytoplasmic maturity that leads to the generation of large polyploid (8N–64N) megakaryocytes. 4. Platelet formation and release of new platelets into the circulation. These mechanisms are significantly different between neonates and adults with thrombocytopenia. In neonates Tpo levels are not as high in thrombocytopenic neonates, particularly in the small for gestational age infants, as those found in children or adults. The number of megakaryocyte progenitors circulating in the peripheral blood of neonates is higher than in children and adults. They give rise to colonies with a greater number of megakaryocytes, and may be more sensitive to Tpo stimulation in comparison with those of children or adults. Megakaryocytes in the neonate are smaller and have lower ploidy, but their cytoplasm reflects that of a mature cell. Last, Tpo effect inhibits megakaryocyte polyploidization in the neonate. Neonates maintain normal platelet counts on the basis of the increased proliferative potential of their megakaryocyte progenitors. Most cases of thrombocytopenia encountered in the NICU are nonimmune and associated with several common neonatal conditions, such as chronic intrauterine hypoxia, sepsis, necrotizing enterocolitis (NEC), and viral infections. Although an indepth review of the particular mechanism underlying the etiology of these nonimmune causes of thrombocytopenia in the neonate is beyond the scope of this review, understanding the differences between neonatal and adult megakaryopoiesis helps us see what predisposes the neonate to develop thrombocytopenia and the potential utility of thrombopoietic growth factors as potential therapeutic interventions in selected neonates. Previous SectionNext Section Platelet Count and Risk of Bleeding Circulating platelets are about one fifth of the diameter of a red blood cell, with a mean volume between 7 and 9 fL. Platelets live for a very short time in the circulation with a half-life of 7 to 10 days. Their primary function is to maintain the integrity of the vascular endothelium and to control hemorrhage from small-vessel injury through the formation of small aggregates or plugs in the microcirculation. Bleeding tendency results when platelets are deficient in number or function. The normal platelet count in newborns and infants is considered to be between 150,000 and 450,000/μL. However, this range of platelet count comes from a limited number of small sample studies of healthy newborns. A study from 18 hospitals in the United States using data from more than 47,000 neonates reported lower limit of platelet ranges from neonates at various gestational ages during their first 90 days after birth of 123,000/μL in late preterm and term neonates and 104,000/μL in infants of less than 32 weeks’ gestation. Whether or not a platelet count below 150,000/μL is considered abnormal, it should always be interpreted within the context of the clinical situation. The tendency to bleed is proportional to the number of platelets within the circulation. As such, there is no risk of bleeding with platelet counts greater than 100,000/μL, minimal or mild risk of bleeding occurs with platelet counts between 20,000 and 100,000/μL, the risk is moderate with platelet counts below 20,000/μL, and the risk is severe and/or there is spontaneous bleeding with platelets below 5,000/μL. In the neonate, the correlation of platelet count with bleeding has not been established. The trauma and the stress of birth can precipitate, although rarely, intracranial or internal bleeding when platelets are below 30,000/μL, and, therefore, clinicians act upon this platelet count to prevent bleeding in the NICU setting. This threshold seems to be higher for preterm infants. Many centers will use a platelet count of 50,000/μL to transfuse preterm infants. However, there is little evidence that this approach will prevent intracranial hemorrhage (ICH). Prospective studies are warranted to establish the most safe and cost-effective threshold at which to transfuse premature infants. The bleeding pattern in the presence of moderate to severe thrombocytopenia is mucocutaneous. The presence of petechiae, bruises, or bleeding from the mucous membranes is characteristic of low platelet counts. In the neonate, intraventricular hemorrhage or intracranial bleed are also possible in the setting of thrombocytopenia. Previous SectionNext Section Diagnostic Approach to Neonatal Thrombocytopenia Multiple disease processes can cause thrombocytopenia. A practical approach to the diagnosis and management of thrombocytopenia in the neonate can be based on the time of onset of thrombocytopenia (early ≤72 hours after birth or late ≥72 hours after birth), gestational age of the patient (term versus preterm), on the underlying mechanism (consumption, increased destruction, decreased production), or on whether the thrombocytopenia is due to maternal or infant factors or individualized to the particular infant. Critical parameters that are common to all these approaches include the severity of thrombocytopenia, the maternal history, the health status of the infant, and the presence or absence of congenital malformations. A simplified approach to the diagnosis of thrombocytopenia in the newborn is presented here. It is based on an algorithm (see Figure) and a table (see Table) that takes the above factors into account, especially the severity of thrombocytopenia and the level of illness of the neonate. View larger version: In this page In a new window Download as PowerPoint Slide Figure. Diagnostic approach to an infant with thrombocytopenia. NAIT=Neonatal alloimmune thrombocytopenia. View this table: In this window In a new window Table. Specific Illnesses and Patterns Associated With Neonatal Thrombocytopenia The sick newborn may become thrombocytopenic from a variety of neonatal complications such as infection, asphyxia, meconium aspiration, respiratory distress syndrome, polycythemia, NEC, and the presence of an indwelling umbilical catheter. In the sick newborn who has severe thrombocytopenia, specific treatment for the underlying condition should be provided and the thrombocytopenia treated symptomatically. In general, mild to moderate thrombocytopenia with a platelet count between 50,000 and 149,000/μL in a healthy newborn that occurs in the first 72 hours after birth is associated with a maternal history of placental insufficiency. These infants will recover a normal platelet count within 10 days and require only close observation. In sick newborns without evidence of placental insufficiency, evaluation for sepsis is warranted in addition to initiation of broad spectrum antibiotics. When the newborn’s underlying condition improves, the thrombocytopenia should also improve, usually within 5 to 7 days. Persistent thrombocytopenia should alert the physician to look for other causes. Patients with severe thrombocytopenia or a platelet count lower than 50,000/mL should be evaluated for sepsis, disseminated intravascular coagulation (DIC), or neonatal alloimmune thrombocytopenia (NAIT). If any of those are present no additional evaluation is required. In newborns without signs of sepsis, additional evaluation must be pursued and must include: (1) maternal history of thrombocytopenia, (2) detailed familial history of thrombocytopenia, (3) detailed physical examination with special attention to the upper extremities, dysmorphic features suggestive of a congenital anomaly or a particular syndrome, such as thrombocytopenia-absent radii syndrome, Fanconi anemia, trisomy 13, 18, 21, or Turner syndrome. In newborns with a negative maternal or familial history and an unrevealing physical examination, other infections, such as toxoplasmosis, other viruses and syphilis, rubella, cytomegalovirus, and human immunodeficiency virus complex; human immunodeficiency virus infection; or enteroviruses, should be considered. In addition, catheter-related thrombosis, chromosomal anomalies, and inborn errors of metabolism, especially propionic acidemia and methylmalonic acidemia, should be considered. The most common etiology of severe thrombocytopenia in an otherwise healthy-looking newborn is immune-mediated thrombocytopenia in which there is passage of maternal antibodies from the mother to the fetus. Other rarer disorders, such as vascular tumors or hemangiomas with Kasabach-Merritt phenomenon and renal vein thrombosis, should be investigated. When thrombocytopenia occurs more than 72 hours after birth, it is more likely to be due to bacterial or fungal sepsis and/or NEC. For patients in whom a bacterial or fungal process is excluded as etiology of thrombocytopenia, viral infections such as herpes simplex virus and cytomegalovirus, DIC, catheter-related thrombosis, drug-induced thrombocytopenia, heparin-induced thrombocytopenia, or other inherited disorders should be considered. This group of patients constitutes the most common reason for consultation to the hematology team. Immune-mediated thrombocytopenias should also be considered in this group, in particular, because they tend to worsen in the first few days after birth. The differential diagnosis of immune-mediated thrombocytopenias is between NAIT, maternal autoimmune disorders, or, rarely, maternal drug-induced immune thrombocytopenia. In the following sections we will highlight some of the features of the most common causes of thrombocytopenia in newborns in the three major categories of destruction of platelets by immune mediated process, decreased or abnormal production of platelets owing to inherited disorders, and consumption of platelets related to some acquired disorders. Previous SectionNext Section Immune-Mediated Causes of Neonatal Thrombocytopenia Neonatal Alloimmune Thrombocytopenia NAIT is a rare disorder that presents in an otherwise well-appearing newborn with moderate to severe thrombocytopenia. NAIT occurs when fetal platelets contain an antigen inherited from the father, a human platelet antigen (HPA) that the mother lacks. Fetal platelets that cross the placenta into the maternal circulation trigger the production of maternal antiplatelet antibodies against the foreign antigen. These antibodies cross the placenta into the fetal circulation and destroy platelets resulting in fetal and neonatal thrombocytopenia. There are sixteen HPAs identified but only three cause 95% of the NAIT cases (HPA-1a, HPA-5b, and HPA-15b). Feto-maternal incompatibility for HPA1a is responsible for 75% of cases in whites. HPA-1a incompatibility occurs in 1:350 pregnancies, although thrombocytopenia develops in only 1:1,000 to 1,500 pregnancies. Infants with NAIT will have symptoms of mucocutaneous bleeding but will look otherwise healthy. Typically, platelet count falls below 50,000 in the first few days after birth, but then rises as the alloantibody concentration declines, which usually happens in 1 to 4 weeks, the expected half-life of immunoglobulin G. This immune-mediated platelet disorder is the equivalent to Rh sensitization of red blood cells with the only difference that NAIT often develops in the first pregnancy of an at-risk couple. The most serious complication of NAIT is ICH. This may occur in as many as 10% of the cases, and up to 50% of the time it happens before birth. All infants with severe thrombocytopenia due to NAIT should have a cranial ultrasound to look for evidence of ICH. If available, the best alternative to treat these patients is the use of HPA-1a–negative platelets from the blood bank. Random donor platelets, even though they are likely to have the target antigen, are effective in treating the infant with severe thrombocytopenia. Platelets obtained from the mother are also effective, but these platelets need to be washed to minimize the presence of the circulating anti-HPA-1a antibodies. Specific platelet antigen and antibody testing are not readily available at all centers but can be requested at major referral laboratories. The administration of intravenous immunoglobulin (IVIG) may be helpful and represents another alternative treatment but less effective than platelet transfusions. Once an infant is diagnosed with NAIT, it is important to carry out a complete diagnostic plan with genotyping of mother and father to be able to provide genetic counseling. In subsequent pregnancies, if the father is a homozygote for the affected antigen, therapy should be started as early as the 13th week of pregnancy with weekly IVIG with or without prednisone, depending on the severity of the previously affected infant or if there was a history of ICH. If the father is a heterozygote for the antigen, the risk of the infant must be determined by molecular analysis of fetal cells circulating in the maternal blood, if available, or by invasive procedures: chorionic villi biopsy or amniocentesis, which both carry significant risks to the pregnancies, to establish if the fetus is affected. Neonatal Autoimmune Thrombocytopenia Neonatal autoimmune thrombocytopenia, in contrast to NAIT, is caused by the passage of maternal antibodies that react with both maternal and infant platelets, and therefore both mother and infant are affected. This disorder occurs in maternal autoimmune disorders such as immune thrombocytopenic purpura or systemic lupus erythematosus. It occurs in 1 to 2:1,000 pregnancies. The diagnosis becomes obvious from a maternal history of thrombocytopenia. Transplacental passage of maternal autoantibodies in this setting is much less of a clinical problem than NAIT. It is important to note that the maternal history is not always positive, because there are many thrombocytopenic mothers who would be asymptomatic and therefore unaware of their own disorder. All neonates of mothers with autoimmune diseases should have their platelet count determined at birth. In most cases, the platelet count rises spontaneously by day 7. In cases of severe thrombocytopenia, treatment with IVIG is recommended. The presence of unexplained thrombocytopenia in a newborn that is suggestive of autoimmune destruction and in whom NAIT has been excluded should trigger evaluation for the presence of an autoimmune disorder in the mother, because neonatal thrombocytopenia can sometimes be the presenting sign of maternal disease. Previous SectionNext Section Inherited Thrombocytopenias Aneuploidies Thrombocytopenia is seen in neonates with trisomy 13, 18, and 21, and also with Turner syndrome. The exact mechanism of thrombocytopenia is unknown, but may be due to reduced platelet production and the pathogenesis may be similar to that seen in chronic fetal hypoxia. Bernard-Soulier Syndrome Bernard-Soulier syndrome (BSS) is a moderate to severe platelet function defect characterized by mild thrombocytopenia, giant platelets, and mucosal type bleeding. It is a very rare disorder with an incidence of 1:1,000,000. It may present in the neonatal period, although bleeding is not usually severe. It is a qualitative platelet disorder with a defect in the von Willebrand factor receptor, the glycoprotein (GP) complex GP Ib–IX– V. The defect in BSS is in the GPIbα gene, and also in the GPIbβ and GPIX genes, mapped to chromosome 17, chromosome 22, and chromosome 3, respectively. Defect in chromosome 22 and abnormality in GPIbβ explains why infants with DiGeorge syndrome and cardiac disease may develop severe bleeding due to a coexisting BSS. The diagnosis of BSS is confirmed by the absence of CD41a or PGPIb–IX–V by flow cytometry. Treatment for BSS is mostly supportive and with platelet transfusion for lifethreatening bleeding. Mothers with BSS may develop alloantibodies against GPIb–IX– V that can cross the placenta, and, therefore, their offspring can develop NAIT due to the passage of alloantibodies against GPIb–IX–V antigens. Wiskott-Aldrich Syndrome Small platelets on peripheral blood film and/or a low mean platelet volume may indicate Wiskott–Aldrich syndrome (WAS). WAS is due to mutations in the WAS protein gene on the short arm of the X chromosome. Mutations in this gene have been isolated to Xp11.23. WAS is characterized by microthrombocytopenia, eczema, and recurrent bacterial and viral infections. Most cases will not present during the neonatal period unless there is a known family history. The disorder usually presents in the first year after birth with bleeding symptoms. Bleeding is due to abnormal platelet function, reduced platelet survival, and thrombocytopenia. X-linked thrombocytopenia is a part of the spectrum of WAS. These patients have thrombocytopenia and small platelets as the major manifestations of their disease. However, the difference is in the variable and less severe degrees of eczema and immunodeficiency. Fanconi Anemia Fanconi anemia (FA) can present in the newborn with persistent thrombocytopenia. It is, in most cases, an autosomal recessive disorder. The infant may present with thrombocytopenia alone, pancytopenia, or with dysmorphic features only. The associated congenital abnormalities of hypopigmented and hyperpigmented skin lesions, microcephaly, small size, urinary-tract abnormalities, and upper-extremity radial-side abnormalities involving the thumb, should alert the physician to the possibility of FA. The cross-linking agent diepoxybutane has been used effectively to diagnose FA and is the standard diagnostic test. Treatment is rarely necessary in the neonatal period. Thrombocytopenia Absent Radii Syndrome This syndrome is characterized by the bilateral absence of the radii and thrombocytopenia. The inheritance pattern of the thrombocytopenia absent radii (TAR) syndrome is autosomal recessive. Both boys and girls are affected, but there is a predominance of girls. The onset of symptoms usually occurs very early in life. Half of the patients have onset of hemorrhagic manifestations in the first week after birth, and most develop thrombocytopenia by 4 months of age. In contrast to infants with FA, in patients with TAR syndrome both thumbs are present. The prognosis in the TAR syndrome is dependent on the severity of the hemorrhagic manifestations. If the patient survives the first year after birth, the hemorrhagic manifestations resolve, because the platelet count spontaneously improves to low normal levels which are then maintained. Treatment is supportive with platelet transfusions indicated only in the event of active bleeding. Congenital Amegakaryocytic Thrombocytopenia Congenital amegakaryocytic thrombocytopenia (CAMT) is a rare recessive autosomal disorder presenting during the neonatal period with thrombocytopenia. Most affected infants have petechiae or other evidence of bleeding. Physical anomalies are present in approximately 50% of the patients. CAMT typically presents with isolated thrombocytopenia; however, 50% of the patients will later progress to aplastic anemia during infancy or early childhood. The bone marrow shows decreased to absent megakaryocytes with normal erythroid and myeloid precursors. Bleeding episodes in neonates with CAMT are treated by platelets transfusions, but stem cell transplantation is the definitive form of therapy for this disorder. There is another variant of amegakaryocytic thrombocytopenia with radioulnar synostosis. Patients with amegakaryocytic thrombocytopenia with radioulnar synostosis have a mutation in the HOXA11 gene that distinguishes them from TAR syndrome, and from CAMT in which the mutation is located in the cMPL gene. Giant Platelet Syndromes Giant platelet syndromes may present in the neonatal period. The characteristic of these disorders is not only a reduced platelet count, but the appearance of large platelets on the peripheral smear. Several of the rare giant platelet syndromes present in the fetus, including the May-Hegglin anomaly, which is characterized by the presence of leukocyte Dohle-like inclusion bodies. This could be a rare cause of fetal or neonatal ICH. The defect in May-Hegglin anomaly is in the MY-H9 gene on chromosome 22q. This mutation is also found in other giant platelet syndromes such as Fletcher syndrome, where leukocyte inclusions are absent and there is association of sensorial hearing loss, nephritis and cataracts, and Epstein syndrome, in which there are no leukocyte inclusions but there is association to hearing loss and nephritis without cataracts. Other macrothrombocytopenias are a group of heterogeneous disorders that include functional platelet disorders like Bernard-Soulier syndrome that was previously described, gray platelet syndrome in which there is lack of platelet granules, and Jacobsen-Paris-Trousseau syndrome that is associated with psychiatric problems or mental retardation. Previous SectionNext Section Consumptive Causes of Thrombocytopenia in the Neonatal Period Kasabach-Merritt Phenomenon This is an important cause of thrombocytopenia in the newborn. It typically presents with profound thrombocytopenia, microangiopathic anemia, and DIC in association with a vascular malformation. The diagnosis is obvious when the vascular anomaly is cutaneous, but it may be more challenging with the presence of vascular anomalies with visceral involvement. The thrombocytopenia is due to the trapping and consumption of platelets in the endothelium of the abnormal blood vessels. The treatment of these lesions requires supportive treatment with plasma and platelet transfusion if DIC is present. Some of these vascular malformations with aggressive behavior may need treatment with steroids, interferon, vincristine, and other chemotherapy agents. More recently, the use of the angiogenesis inhibitor bevacizumab and the use of the mTOR inhibitor, rapamycin, have shown some activity; however, further studies are necessary before recommending therapy with these agents. Thrombotic Disorders Acquired thrombotic events in the NICU have increased over the past several years, mainly because of the high complexity of the patients cared for in the NICU requiring indwelling catheters and being at risk for several factors that predispose them to secondary thrombotic events. The use of heparin flushes to maintain the patency of indwelling catheters is also a risk for the development of heparin-induced thrombocytopenia that is associated with arterial thrombosis. Inherited deficiency of ADAMTS13, the cleaving protease of von Willebrand factor, that causes thrombotic thrombocytopenic purpura may present in the newborn period. Renal vein thrombosis should also be considered in the differential diagnosis of patients with thrombocytopenia and renal failure. Thrombocytopenia is part of the clinical presentation of anticoagulant factor deficiencies, and these deficiencies should be considered in the differential diagnosis of thrombocytopenia. However, the severe form of these deficiencies presents with purpura fulminans and diffuse thromboses and not just isolated thrombocytopenia. Previous SectionNext Section Conclusions Thrombocytopenia is a common problem in the newborn. The differential diagnosis of thrombocytopenia in the neonate can be simplified when taking into account the severity of the thrombocytopenia and the clinical appearance of the neonate. Most episodes of thrombocytopenia in the newborn occur after the first 72 hours after birth and are most commonly caused by infectious process. Persistent thrombocytopenia, or thrombocytopenia that does not respond to adequate treatment of the presumed etiology of the low platelet count, deserves further investigation to look for some of the rare causes of thrombocytopenia in neonates including immune-related disorders, inherited thrombocytopathies and other acquired causes of platelet consumption. American Board of Pediatrics Neonatal–Perinatal Content Specifications Know the normal pattern of platelet production and maturation. Know the causes and pathophysiology of neonatal thrombocytopenia and thrombocytosis. Know the clinical and laboratory manifestations and management of neonatal thrombocytopenia and thrombocytosis. Previous SectionNext Section Footnotes Author Disclosure Drs Fernández and de Alarcón have disclosed no financial relationships relevant to this article. This commentary does contain a discussion of an unapproved/investigative use of a commercial product/device. Abbreviations: BSS; Bernard Soulier syndrome CAMT; congenital amegakaryocytic thrombocytopenia DIC; disseminated intravascular coagulation FA; Fanconi anemia GP; glycoprotein HPA; human platelet antigen ICH; intracranial hemorrhage IVIG; intravenous immunoglobulin NAIT; neonatal alloimmune thrombocytopenia NEC; necrotizing enterocolitis NICU; neonatal intensive care unit TAR; thrombocytopenia absent radii Tpo; thrombopoietin WAS; Wiskott-Aldrich syndrome Copyright © 2013 by the American Academy of Pediatrics Previous Section Suggested Reading 1. 1. Chakravorty S, 2. Roberts I . How I manage neonatal thrombocytopenia. Br J Haematol. 2012;156(2):155– 162 CrossRefMedline 2. 1. Drachman JG . Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood. 2004;103(2):390–398 Abstract/FREE Full Text 3. 1. 2. 3. 4. Ferrer-Marin F, Liu ZJ, Gutti R, Sola-Visner M . Neonatal thrombocytopenia and megakaryocytopoiesis. Semin Hematol. 2010;47(3):281–288 CrossRefMedline 4. 1. 2. 3. 4. Hammill AM, Wentzel M, Gupta A, et al . Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024 CrossRefMedlineWeb of Science 5. 1. Nurden P, 2. Nurden AT . Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99(2):253–263 MedlineWeb of Science 6. 1. Risson DC, 2. Davies MW, 3. Williams BA . Review of neonatal alloimmune thrombocytopenia. J Paediatr Child Health. 2012;48(9):816–822 CrossRefMedline 7. 1. Sola-Visner M, 2. Sallmon H, 3. Brown R . New insights into the mechanisms of nonimmune thrombocytopenia in neonates. Semin Perinatol. 2009;33(1):43–51 CrossRefMedlineWeb of Science Hemolytic Disease of the Fetus and Newborn 1. Mary Beth Ross, MD*, and 2. Pedro de Alarcón, MD† + Author Affiliations 1. * Assistant Professor of Pediatrics, University of Illinois College of Medicine at Peoria and Children’s Hospital of Illinois, Peoria, IL. 2. †William H. Albers Professor and Chair, Department of Pediatrics, University of Illinois College of Medicine at Peoria and Children’s Hospital of Illinois, Peoria, IL. Next Section Abstract Hemolytic disease of the fetus and newborn (HDFN) is the result of immune-mediated destruction of fetal or newborn red blood cells when such cells contain antigens that are not present in the maternal blood. HDFN is now the preferred term that replaces the historic term erythroblastosis fetalis. Sensitization of the mother to fetal-newborn red blood cells requires fetomaternal hemorrhage in most cases except in ABO incompatibility where naturally occurring antibodies against A and B antigens are present in mothers with O blood type. The most common antigen involved in HDFN is Rhesus D. Kell 1 HDFN is rare but commonly associated with severe anemia and lower titers of anti-Kell antibodies in maternal serum in severely affected infants. Prevention of Rhesus D HDFN with anti-D immunoglobulin during pregnancy, delivery, and fetalmaternal events that predispose to fetomaternal hemorrhage, have markedly decreased the incidence of the disorder but may not be available in low-income countries. An algorithm is available to manage affected pregnancies by using antibody titers, fetal middle cerebral artery velocities, intrauterine transfusions, and timed delivery. Infants who have mild to moderate anemia may tolerate normal labor, but severely affected infants may require transfusion or exchange transfusions at birth, and the delivery team needs to be prepared. Delayed anemia in the transfused infants is still a concern, and the infants need to be closely followed after delivery. Phototherapy has largely replaced exchange transfusion in the management of hyperbilirubinemia. With appropriate early detection and multidisciplinary planning, infants who have HDFN can be delivered in a timely manner with appropriate planning for postnatal resuscitation and postnatal therapy resulting in good neonatal outcomes. Previous SectionNext Section Objectives After completing this article, readers should be able to: 1. Recognize the symptoms and signs associated with hemolytic disease of the fetus and newborn (HDFN). 2. Understand the pathophysiology of HDFN. 3. List the red blood cell antigens most commonly associated with HDFN. 4. Identify the fetus at risk for HDFN. 5. Discuss the clinical management of fetus/newborn affected by HDFN. Previous SectionNext Section Introduction Advances in prevention and detection have markedly decreased the incidence of hemolytic disease of the fetus and newborn (HDFN). The disorder, caused by red blood cell (RBC) incompatibility between infant and mother, and its multiple clinical manifestations were first brought together in 1932 in the landmark article by Dr Louis K. Diamond, where he coined the term erythroblastosis fetalis, based on the morphology of the peripheral blood smear seen in infants who have severe diseases. HDFN is now the preferred term encompassing all infants who have alloimmune hemolysis, whether or not erythroblasts are present. The work of Landsteiner and other investigators defined RBC antigens and their role in transfusion reaction by 1940, and Levine et al in 1941 gathered enough cases to document that HDFN was indeed caused by blood group incompatibility between a mother and her infant. HDFN is the result of immune destruction of fetal or newborn RBCs. Maternal antibodies develop when fetal RBCs express cell surface antigens that are not present on the maternal RBCs. For this process to occur, fetal RBCs must enter the maternal circulation secondary to fetomaternal hemorrhage (FMH). The exception to this rule is ABO incompatibility because type O mothers have naturally occurring anti-A and anti-B antibodies. HDFN should be considered in the differential diagnosis of postnatal early, severe, or prolonged jaundice. It also should be considered in the differential diagnosis of neonatal anemia, in particular, if it is severe or associated with hydrops fetalis. The presence of maternal RBC antibodies and/or a positive direct antibody testing in the infant are diagnostic for HDFN. A comprehensive maternal history is essential for the proper diagnosis of HDFN. Particular emphasis should be placed on uncovering maternal events that predispose to FMH. A maternal history of a previous pregnancy, particularly a complicated pregnancy, history of hydrops fetalis, a miscarriage, early termination of pregnancy, blood transfusion, or clinical documentation of FMH, should alert the clinician to the possibility of HDFN in an infant with anemia and/or jaundice. The greatest advances in this disorder have been the introduction of very effective prevention strategies, the aggressive use of phototherapy for jaundice, and the introduction of noninvasive techniques to monitor the affected fetus. Previous SectionNext Section Differential Diagnosis The two main signs of HDFN are anemia and hyperbilirubinemia. The anemia is hemolytic and the bone marrow is reactive with reticulocytosis and often presents immature RBCs, erythroblasts, in the peripheral blood; hence, the original term erythroblastosis fetalis was given to the disorder. Other types of non–immune-mediated hemolysis can result in anemia and hyperbilirubinemia. These include RBC membrane defects such as hereditary spherocytosis and hereditary elliptocytosis. Although these disorders are hereditary, their phenotype is variable, and the RBC defects may go undiagnosed well into adulthood. Clues that can be suggestive of undiagnosed RBC membrane defects include family members who are intermittently jaundiced, intermittently have scleral icterus, or intermittently have dark urine. These episodes generally occur in association with acute illness. Family members who have a history of splenectomy unrelated to trauma or early gallbladder disease may suggest an undiagnosed inherited RBC membrane defect. Hemoglobinopathies also may contribute to neonatal hemolysis. Hemoglobinopathy screening in the United States has been available in most states for over a generation, and today all 50 states screen for these disorders and they are diagnosed at birth. Nonetheless, recent immigrants to the United States may have previously undiagnosed hemoglobinopathies. Review of the blood smear, newborn screening, confirmatory hemoglobin electrophoresis, and potentially maternal and paternal hemoglobin electrophoresis should clarify this diagnosis. RBC enzyme defects such as glucose-6-phophate dehydrogenase can lead to hemolysis and anemia. Significant and prolonged hyperbilirubinemia and even kernicterus have been described in infants who have glucose-6-phophate dehydrogenase deficiencies, particularly in the Philippines, Africa, and Greece. Hemolysis can occur from disorders of glycolysis such as pyruvate kinase deficiency. Here too, family history and ethnic background can be key factors in identifying the risk for RBC enzyme defects. Previous SectionNext Section Mechanisms of Maternal Exposure All individuals with blood type O naturally express antibodies to A and B RBC surface antigens. No previous exposure to blood group antigens is necessary for antibody development. These antibodies are immunoglobulin G type and cross the placenta. Individuals who have blood type A have antibodies against type B and vice versa. However, these antibodies are predominantly immunoglobulin M class and do not cross the placental barrier. For all other blood group antigens, maternal exposure to blood group antigens is necessary for the development of antibodies. The most frequent cause of maternal sensitization is FMH. FMH can occur in the first trimester, but is most common in the third trimester. Numerous fetal events or procedures may be associated with previously underappreciated risk for FMH. See the Table for a listing of events that may be associated with FMH. View this table: In this window In a new window Table. Mechanisms of Maternal Exposure to Red Blood Cell Antigens Women who have a known history of transfusion represent only a small proportion of all pregnant women. However, women who have a previous transfusion history represent half of the pregnancies affected by non-Rhesus D (RhD) HDFN. Routine blood typing and cross-match screen for ABO and RhD blood types, but none of the other blood types involve HDFN. As more children survive previously serious childhood illnesses such as cancer and congenital heart disease, we are likely to see an increase in HDFN from maternal transfusion. In fact, the mother may be unaware of her own blood product exposure, because treatment may have occurred during her early childhood. See the Table for a listing of maternal history elements that raise concern for unappreciated exposure to RBC antigens. The ever expanding utilization of reproductive technologies leads to unexpected risks for HDFN. The battle with infertility is highly personal in nature. That, combined with our fragmented health-care system and highly mobile society, leads to significant opportunity for utilization of egg donor, sperm donor, or embryo adoption to go undisclosed to the health-care team at the time of delivery. Even when a family is willing to disclose use of egg donor, sperm donor, or embryo adoption, ABO blood type of the donors may or may not be known to the pregnant woman. This circumstance can set up otherwise uninheritable combinations of RBC antigens. For example, an O− (negative) mother with an O− (negative) husband could deliver an AB+(positive) infant who is at risk for HDFN from ABO and Rh mismatch. Previous SectionNext Section Red Blood Cell Antigens Most Frequently Involved in Hemolytic Disease of the Fetus and Newborn Rhesus (RhD) incompatibility is the best described cause of HDFN. Despite international efforts aimed at prevention of HDFN in infants of Rh-negative mothers, Rh remains the most commonly identified RBC antigen causing HDFN. RhD-negative denotes the lack of D antigen on the RBC surface. Eleven percent to 35% of white populations are RhD-negative because of gene deletion. In contrast, most East Asian and African populations that lack RBC surface expression of RhD have a grossly intact gene. In Africans, a 37-bp insertion results in the insertion of a stop codon leading to a prematurely shortened protein product. A special circumstance exists with an RhD variant called weak D. There is both an altered protein sequence and decreased cell surface protein expression. Serologic testing will identify this person as RhD-negative. Although more sensitive testing can demonstrate the presence of the protein on the cell surface, importantly, these apparently RhD-negative mothers will not form anti-D antibodies even if they are transfused with RhD-positive blood. The second most common RBC antigen associated with HDFN is the ABO blood group. HDFN due to an ABO blood group mismatch occurs almost exclusively in infants with mothers of blood type O. Hemolysis is more common with anti-A than with anti-B. The clinical presentation for HDFN due to ABO blood group is predominantly hyperbilirubinemia without severe anemia. Phototherapy is generally sufficient for most of these infants. Another major cause of HDFN is Kel1 antigen of the Kell antigen system. Kell is a glycoprotein containing 15 antigens and their antithetical variants. It is the first erythroid-specific antigen known to be expressed during erythroid development. Clinically, anti-Kel1 HDFN is manifest by more severe anemia and reticulocytopenia. Hyperbilirubinemia is less severe than with other RBC antigens. Anti-Kel1 antibody is rare and is only found in 0.1% of pregnant women. However, most of these women have developed antibody because of previous transfusion exposure. Fortunately, only 9% of people of European descent and 2% of people of African descent express Kel1. Because of the early erythroid lineage expression of Kell when Kel1 is present, antiKel1 is associated with a lower critical antibody titer (1:8) than anti-RhD or antibodies to other RBC antigens. A variety of other RBC antigens have been described as causing HDFN. For a detailed listing of RBC antigens, the severity of HDFN associated with each antigen, and reference citation, see Table 6.1 in de Alarcon, Werner, and Christensen’s Neonatal Hematology. Previous SectionNext Section Clinical Management: Prevention of Hemolytic Disease of the Fetus and Newborn Due to Rhesus D The first approach to prevention of HDFN due to an RhD-positive fetus born to an RhD-negative mother is administration of one postnatal dose of anti-RhD. Between 1968 and 1983, both maternal immunization and perinatal infant deaths were reduced by 90%. Subsequently, clinical trials were performed demonstrating that the administration anti-RhD at 28 weeks’ gestation in addition to the postnatal dose could further decrease the frequency of immunization from the remaining 2% down to 0.2%. Similarly, an appreciation rose for other events that led to FMH effectively immunizing the mother further against RhD. We now know that fetal and pregnancy events such as ectopic pregnancy, spontaneous or induced abortion, fetal demise, and maternal abdominal trauma can result in maternal immunization from FMH. Invasive medical procedures such as amniocentesis, cordocentesis, and chorionic villus sampling are associated with an increased risk of immunization to RhD. Recommendations are now in place for immune prophylaxis in the event of planned or unscheduled events that may increase FMH and therefore increase maternal exposure to RhD. For a listing of such events, see the Table. Anti-RhD prophylaxis policies vary slightly between developed countries (such as the United States, England, Mexico, Canada, and Australia). In third world countries, access to anti-RhD may still be extremely limited both owing to medical infrastructure reasons as well as socioeconomic reasons such as minimal prenatal care and delivery at home. In the United States, the Preventative Services Task Force current consensus recommendations are available at http://www.uspreventiveservicestaskforce.org (then search the site for “Rh incompatibility”). Current US recommendations include the proposal that all pregnant women should have antibody screening and RBC antigen typing for ABO and RhD at the first prenatal visit. RhD-negative mothers with no antibody present at the first screen should have repeat screening at 24 to 28 weeks. If they remain negative, they should receive 1,500 IUs (= 300 μg) anti-RhD immunoglobulin at 28 weeks’ gestation unless the father of the infant is also known be RhD-negative. Second, women at high risk for FMH, such as previous transfusion, obstetrical complications in which anti-RhD was not administered, or the woman had sustained injuries, should continue to be screened for antibody development into the second and third trimesters. An additional dose of anti-RhD is recommended for women experiencing obstetrical complications with a risk for FMH or undergoing diagnostic testing that can increase FMH. This anti-RhD dose is trimester-dependent. For events in the first trimester, the recommended dose is 250 IUs (= 50 μg). For events in the second or third trimester, the recommended dose is 1,500 IUs (= 300 μg). Last, the postnatal dose of 1,500 IUs is administered to all RhD-negative mothers. This dose should prevent immunization when as much as 15 mL of newborn blood enters the maternal circulation. Women experiencing high-risk problems or maneuvers, such as abruption, manual removal of the placenta, or multiple infant gestations may be at risk for higher volume FMH. An effort should be made to quantitate the FMH and administer additional anti-RhD, if necessary (when estimated to be >15 mL). Previous SectionNext Section Clinical Management: Identifying Pregnancies at Risk for Hemolytic Disease of the Fetus and Newborn Monitoring is dependent upon previous pregnancy history, whether the infant from a previous pregnancy was clinically affected, which RBC antigen is involved, what is the father’s genotype and phenotype, how high is maternal antibody, and whether there is clinical evidence of fetal anemia. The first level of monitoring is phlebotomy for serial determination of anti–RBC antigen antibodies or indirect antiglobulin testing (IAT). An algorithm has been developed by Moise for monitoring of pregnancy in an RhDnegative woman with an RhD-positive fetus or RhD-positive father. This algorithm can also be applied to assessing risk for fetal anemia due to other RBC antigens. A first pregnancy of an RhD-negative mother is monitored by repeated anti–RBC antibody titers. If the titer remains low, serial monitoring continues and the infant is delivered at term. If the titer crosses above a critical threshold, then infants are monitored by serial fetal middle cerebral artery (MCA) velocities. An increased MCA velocity correlates well with fetal anemia. For pregnancies with previously affected infants, the current fetus is monitored with serial MCA velocities by using Doppler ultrasound to monitor for development of fetal anemia. Previous SectionNext Section Clinical Management: Intrauterine Once a pregnancy is identified as being at risk because of positive or rising IAT, serial Doppler ultrasound MCA velocities are utilized to monitor for fetal anemia. If mild anemia is detected, serial monitoring by ultrasound continues until there is adequate lung maturity or term delivery. Where severe anemia is suspected, cordocentesis may be utilized to confirm severe anemia (hematocrit <30% or hemoglobin <10 g/dL). In the event of severe anemia, an intrauterine transfusion may prevent progression to a severely ill, hydropic infant. Packed red blood cells (pRBCs) that are negative for the RBC antigen involved in the HDFN are used for transfusion. Additional pRBC specifications include leukodepletion, cytomegalovirus-negative donor, and irradiated products to prevent transfusionassociated graft versus host disease. Previous SectionNext Section Clinical Management: Postnatal Delivery, either preterm or term, should be scheduled in a perinatal level 3 center with interdisciplinary obstetric, maternal-fetal, and pediatric services available. Assessment of fetal lung maturity and risk for subsequent respiratory distress must be taken into account in preparation for delivery. This includes consideration of glucocorticoids to accelerate lung maturity, when appropriate, and planning for personnel and equipment for resuscitation of the infant experiencing respiratory distress. A mildly or moderately anemic fetus will often tolerate labor adequately. A severely anemic fetus may not. ABO matched (if ABO typing has been done with previous cordocentesis) or O-type blood that is also negative for the antigen responsible for HDFN should be available to the resuscitating team in the delivery room. This pRBC product should be leukodepleted, cytomegalovirus-negative, and irradiated. The most severely ill infants may require an exchange transfusion, which involves replacing the native infant RBCs with appropriately antigen-negative RBCs to prevent further hemolysis. This is accomplished by replacing a total of 25 to 50 mL/kg pRBCs. Once vascular access has been established, 5 mL/kg aliquots can be removed and replaced over several minutes. This cycle is repeated until the targeted volume is replaced. Many anemic but more stable infants may be transfused with ABO-matched pRBCs that are negative for the antigen contributing to the HDFN at 10 mL/kg over a 2- to 3-hour period; 3 mL/kg pRBCs are needed to increase the hemoglobin 1 gm/dL (hematocrit 3%). Exchange transfusion was the mainstay of therapy early in management of RhD HDFN to minimize kernicterus from hyperbilirubinemic infants. Now, many hyperbilirubinemic infants who have HDFN will respond adequately to early phototherapy with blue light “bilirubin lights,” often in combination with “bilirubin blankets.” These infants may only require early phototherapy in combination with transfusion to address mild to moderate anemia. HDFN from some RBC antigens may result in delayed or prolonged postnatal anemia. Therefore a pediatric hematologist should be consulted to help monitor for the need for delayed or repeated pRBC transfusion. Previous SectionNext Section Conclusions RhD remains the most significant antigen contributing to HDFN. However, prenatal anti-D prophylaxis has significantly decreased the incidence of severe HDFN. At this time, antibody prophylaxis is only available for HDFN due to RhD. Appropriate prenatal screening with IAT and Doppler ultrasound has greatly improved infant outcomes by allowing early identification of pregnancies at risk for HDFN. With appropriate early detection and multidisciplinary planning, these infants can be delivered in a timely manner with appropriate planning for postnatal resuscitation and postnatal therapy resulting in good neonatal outcomes. American Board of Pediatrics Neonatal–Perinatal Content Specifications Know the diagnostic evaluation and perinatal management of fetal-maternal blood group incompatibility. Know the etiology and pathophysiology of hemolytic anemias in the neonate. Know the clinical and laboratory features of hemolytic anemia in the neonate. Know the management of hemolytic anemia in the neonate. Previous SectionNext Section Footnotes Author Disclosure Drs Ross and de Alarcón have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device. Abbreviations: FMH; fetomaternal hemorrhage HDFN; hemolytic disease of the fetus and newborn IAT; indirect antiglobulin testing MCA; middle cerebral artery pRBC; packed red blood cell RBC; red blood cell RhD; Rhesus D Copyright © 2013 by the American Academy of Pediatrics Previous Section Suggested Reading 1. 1. Basu S, 2. Kaur R, 3. Kaur G . Hemolytic disease of the fetus and newborn: Current trends and perspectives. Asian J Transfus Sci. 2011;5(1):3–7 CrossRefMedline 2. 1. Brinc D, 2. Lazarus AH . Mechanisms of anti-D action in the prevention of hemolytic disease of the fetus and newborn. Hematology (Am Soc Hematol Educ Program). 2009:185–191 Search Google Scholar 3. 1. Diamond LK, 2. Blackfan KD, 3. Baty JM . Erythroblastosis fetalis and its association with universal edema of the fetus, icterus gravis neonatorum and anemia of the newborn. J Pediatr. 1932;1(3):269–309 CrossRef 4. 1. Illanes S, 2. Soothill P . Noninvasive approach for the management of hemolytic disease of the fetus. Expert Rev Hematol. 2009;2(5):577–582 CrossRefMedline 5. 1. Moise KJ Jr . Management of rhesus alloimmunization in pregnancy. Obstet Gynecol. 2008;112(1):164–176 CrossRefMedlineWeb of Science 6. 1. Osaro E, 2. Charles AT . Rh isoimmunization in Sub-Saharan Africa indicates need for universal access to anti-RhD immunoglobulin and effective management of D-negative pregnancies. Int J Womens Health (Larchmt). 2010;2:429–437 Search Google Scholar 7. 1. 2. 3. 4. Ross ME, Waldron PE, Cashore WJ, de Alarcon PA . Hemolytic disease of the fetus and newborn. In: de Alarcon PA, Werner EJ, Christensen RD, eds. Neonatal Hematology, Pathogenesis, Diagnosis, and Management of Hematologic Problems. 2nd ed. Cambridge, UK; Cambridge University Press; 2013 Search Google Scholar 8. 1. Stockman JA III . Overview of the state of the art of Rh disease: history, current clinical management, and recent progress. J Pediatr Hematol Oncol. 2001;23(6):385– 393 CrossRefMedlineWeb of Science Preeclampsia: Pathophysiology, Management, and Maternal and Fetal Sequelae 1. Mollie McDonnold, MD, and 2. Gayle Olson, MD + Author Affiliations 1. University of Texas Medical Branch, Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, Galveston, TX. Next Section Abstract Preeclampsia is a unique, complicated problem of pregnancy that is prevalent worldwide. The maternal effects of severe disease may involve multiple organ systems. Consequences of disease for the infant include possible prematurity, fetal growth restriction, placental abruption, or intrauterine fetal demise. In addition, long-term effects of disease have been studied in both mothers and children. Although the exact cause of preeclampsia is not fully understood, increasing evidence points to abnormal placentation and an imbalance of antiangiogenic factors. Specifically, soluble Fms-like tyrosine kinase-1 has been investigated as the link between poor placental invasion and maternal disease. Clinically, maternal disease is defined as the presence of elevated blood pressure after 20 weeks’ gestation and proteinuria. The presence of severe symptoms or abnormal laboratory test results separate mild and severe disease. Studies have shown that delivery should occur at 37 weeks’ gestation with mild disease and 34 weeks’ gestation with severe disease. In early-onset severe disease, expectant management with close monitoring is possible if maternal and fetal status remain stable. Pathophysiology, diagnosis criteria, management, and possible maternal and fetal complications are reviewed. Previous SectionNext Section Practice Gaps 1. The timing of delivery and supportive treatments for mothers with preeclampsia can help to reduce both maternal and neonatal morbidity. 2. Neonates born to mothers who have preeclampsia are at risk not only for morbidities in the newborn period but also for diseases later in life. Previous SectionNext Section Objectives After completing this article, readers should be able to: 1. 2. 3. 4. Review the underlying pathophysiology of preeclampsia. Understand the criteria for the diagnosis of the spectrum of preeclampsia. Review indications and timing for delivery. List short- and long-term neonatal consequences of maternal preeclampsia. Previous SectionNext Section Introduction Preeclampsia is the most common medical complication of pregnancy worldwide, occurring in 3% to 5% of all pregnancies and carrying a perinatal and neonatal mortality rate of 10%. (1) Severe preeclampsia occurs in 0.6% to 1.2% of pregnancies in Western countries, whereas preeclampsia less than 37 weeks’ gestation and severe disease less than 34 weeks’ gestation occur in 0.6% to 1.5% and 0.3% of pregnancies, respectively. (2) Preeclampsia is a multisystem disorder with variable effects on the maternal brain, lungs, kidney, liver, coagulation cascade, and the fetus. It is a disease spectrum whose clinical manifestations vary by gestational age at onset and by severity of symptoms. Mild disease is primarily associated with good outcomes. Conversely, severe disease, especially if early onset, is associated with increased complications of eclampsia; cerebral hemorrhage; renal failure; hepatic failure; HELLP (hemolysis, elevated liver enzymes, and low platelet count syndrome); prematurity; fetal growth restriction; oligohydramnios; placental abruption; and intrauterine fetal demise. The only treatment that has proved effective is timely delivery of the neonate; thus, avoidance of poor maternal outcome often means the burden of morbidity and mortality is placed on the neonate. This review focuses on the pathophysiology, diagnosis, management, and maternal and neonatal outcomes of severe preeclampsia. Previous SectionNext Section Pathophysiology Although the pathophysiology of preeclampsia is not fully understood, it is generally regarded as a two-stage disorder. The first stage consists of reduced placental perfusion, likely due to abnormal implantation and abnormal development of the placental vasculature. (3)(4) Early normal placental development is characterized by invasion of the uterine spiral arteries of the decidua and myometrium by extravillous cytotrophoblasts. These transform the uterine vessels from small and resistant to compliant with high-caliber capacitance. This change allows for the increase in uterine blood flow needed to sustain the fetus in the second half of pregnancy. (4)(5)(6)(7) In addition, there is a rise in oxygen tension, stimulating cytotrophoblasts to downregulate the expression of adhesion molecules characteristic of their epithelial origin and adopt an endothelial surface adhesion phenotype. (5) Figure 1 shows the abnormal events occurring in preeclampsia. The invasion of the arteries is limited to the superficial decidua, leaving the myometrial segments narrow and undilated. (1)(4) This action contributes to a deficient blood supply from the maternal side, resulting in prolonged fetal hypoxia and aberrant formation of the placental vasculature. (4) Without changes in oxygen tension, endothelialization fails to occur. (5) Evidence of placental hypoperfusion and ischemia may be seen on pathological specimens, including acute atherosis, intimal thickening, necrosis, atherosclerosis, endothelial damage, and placental infarction. Although these findings are not universal, they do correlate with disease severity. (1)(7) View larger version: In this page In a new window Download as PowerPoint Slide Figure 1. Abnormal events occurring in preeclampsia. Angiogenic factors (vascular endothelial growth factor [VEGF] and placental growth factor) are highly expressed by invading cytotrophoblasts and are important in the regulation of placental vasculogenesis. (1)(5) An imbalance between these factors and antiangiogenic factors, specifically soluble Fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin, leads to an antiangiogenic state and endothelial dysfunction. (1)(4)(5)(6) Levels of sFlt-1 messenger RNA are upregulated in placentas of preeclamptic patients, both at the time of clinical disease and 2 to 5 weeks before onset, with higher elevations noted in severe preeclampsia, early-onset preeclampsia, and preeclampsia complicated by intrauterine growth restriction. (5) Hypoxia has been shown to increase the expression of sFlt-1 in primary trophoblast cultures of firsttrimester placentas. (5) It is this imbalance in angiogenic factors that seems to be the link between abnormal placentation and the second stage of preeclampsia, the maternal response, which is manifested by widespread inflammation and maternal endothelial cell dysfunction, increased markers of oxidative stress, insulin resistance, reduced immune function, and dyslipidemia. (3)(4)(7) In addition, sFlt-1 antagonizes VEGF, preventing it from stabilizing endothelial cells. Clinically, this action is seen as elevated blood pressure and proteinuria, along with multiorgan system involvement. (1) Fetal complications are also evident at this stage and include prematurity, oligohydramnios, intrauterine growth restriction, placental abruption, and intrauterine demise. It is likely that impaired uteroplacental blood flow or placental infarction plays a role in determining this fetal morbidity. Previous SectionNext Section Screening And Diagnosis Maternal risk factors for preeclampsia include primiparity, multifetal gestations, extremes of maternal age, history of preeclampsia, and medical comorbidities such as obesity, hypercoagulability, chronic hypertension, renal disease, lupus, and diabetes mellitus. (1)(2) Although there is evidence of an imbalance in angiogenic markers, there are currently no clinically available biomarkers for use in screening for preeclampsia. In women with pre-existing hypertension or proteinuria, the diagnosis can be difficult, but worsening symptoms, or the development of other clinical or laboratory findings of severe disease, are suggestive. Therefore, it is standard practice to obtain baseline studies, including 24-hour urine for protein quantification, in women with diabetes, chronic hypertension, or underlying renal disease. Blood pressure is obtained during each prenatal visit. These measurements occur monthly until the end of the third trimester, at which time weekly visits are planned. Additional visits are tailored to pre-existing maternal conditions. Hypertension in pregnancy represents a spectrum of disorders, and criteria for the diagnosis of these disorders are listed in Table 1. Critical to the diagnosis is the gestational age of the pregnancy. Preeclampsia occurs after 20 weeks’ gestation; therefore, increased blood pressure at less than 20 weeks warrants investigation for other disorders. When elevated blood pressure (>140/90 mm Hg) or patient symptoms are noted, additional observation and laboratory studies as listed in Table 1 will be needed. (2)(8) View this table: In this window In a new window Table 1. Diagnostic Criteria Previous SectionNext Section Management The management of preeclampsia consists of reducing maternal and neonatal morbidity by controlling blood pressure levels, preventing eclampsia, and timing delivery appropriately. For those women who have nonsevere disease, treatment of blood pressure is usually not required. Once the diagnosis is made, increased observation begins, the location of which is practitioner dependent. Whether as an inpatient or outpatient, the woman’s blood pressure, laboratory data, fetal growth, and amniotic fluid levels are monitored to look for signs of worsening disease. If findings of severe disease do not develop, expectant management may be undertaken until 37 weeks’ gestation. (9) Delivery by this gestational age has been shown to improve outcomes in women who have nonsevere disease. In the Hypertension and Preeclampsia Intervention Trial At Term (HYPITAT), a randomized controlled study of 756 women presenting with gestational hypertension or preeclampsia between 36 and 41 weeks’ gestation, those who were induced within 24 hours of presentation had a 0.71 relative risk (RR) of experiencing poor maternal outcomes (confidence interval [CI]: 0.59–0.86), defined as maternal mortality; eclampsia; hemolysis, elevated liver enzymes, low platelet count syndrome; pulmonary edema; thromboembolic disease; placental abruption; progression to severe disease; and major postpartum hemorrhage. (10) These patients were compared with those who were expectantly managed until the onset of labor. In the two groups, there was no difference in neonatal morbidity. No fetal deaths were recorded in either group. There were similar rates of Apgar scores less than 7 at 5 minutes, admission to intensive care, and arterial pH less than 7.05. For women who have severe hypertension, obtaining control of maternal blood pressure is necessary to decrease the consequences of acute hypertension, including cerebrovascular accident or myocardial ischemia, but blood pressure should not be reduced so dramatically as to impair uteroplacental perfusion. Persistently severe ranging blood pressures are treated to achieve a target range of systolic blood pressure of 140 to 155 mm Hg and a diastolic blood pressure of 90 to 105 mm Hg. (11) Commonly used agents for acute and chronic control include nicardipine, labetalol, hydralazine, and nifedipine. Magnesium sulfate is a mainstay in the treatment of preeclamptic disorders. The Magpie trial, a double-blind, placebo-controlled study of 10,141 women worldwide, demonstrated a 58% lower risk of eclampsia in women who received magnesium sulfate as opposed to placebo. (12) There was also a 0.67 RR of placental abruption (CI: 0.45– 0.89) associated with magnesium sulfate administration. No differences in maternal morbidity, including respiratory depression or arrest, pneumonia, cardiac arrest, coagulopathy, renal failure, liver failure, pulmonary edema, cerebral hemorrhage, toxicity, induction and length of labor, cesarean delivery rate, retained placenta, blood loss, or transfusion were seen. Subsequently, the use of magnesium sulfate has become standard in cases of severe preeclampsia, both during labor and in the initial postpartum period. The use in nonsevere disease is practice dependent. However, studies have shown that the signs considered to be prognostic for seizures, including headache, epigastric pain, or hyperreflexia, are not reliable, and when the use of magnesium sulfate was limited to only severe cases, the incidence of eclampsia increased by 50%. (8) In the Magpie trial, there was no difference in neonatal morbidity for those fetuses whose mothers received magnesium, including Apgar scores less than 7 at 5 minutes, need for intubation at time of delivery, need for ventilation, abnormal cerebral ultrasound, convulsions, or admission to the NICU. (12) Eclampsia is most likely to occur during intrapartum or immediate postpartum periods when the disease accelerates and is diagnosed when the patient experiences generalized tonic-clonic seizures that cannot be attributed to other causes. (8) It is not uncommon to observe prolonged fetal bradycardia during eclamptic seizures, but fetal status generally improves with maternal stabilization and oxygenation. Thus, it is generally not advised to perform an urgent cesarean delivery during an acute seizure. In women who have severe disease, delivery by 34 weeks’ gestation seems to maximize maternal and neonatal outcomes. When the diagnosis is made remote from this gestational age, management becomes more complex. The administration of corticosteroids for fetal lung maturity is considered if presentation is between 24 and 34 weeks’ gestation for any degree of preeclampsia. At minimum, the goal is to continue observation for 48 hours after the steroids are administered to achieve benefit for the infant. The maximum length of observation is dependent on the severity of the course of preeclampsia. The median latency from time of diagnosis of severe, early-onset disease to delivery is 7 to 14 days. (2) However, in cases of worsening disease, even a 48-hour window of observation may not be attainable. If the disease is ameliorated, observation could continue until 34 weeks’ gestation for those who have severe but stable preeclampsia or 37 weeks’ gestation with nonsevere disease. Timing and indications for delivery are shown in Table 2. (2)(11) Two randomized trials have compared expectant management of severe disease until at most 34 weeks' gestation to delivery immediately following corticosteroid benefit. Immediate delivery was associated with higher rates of respiratory distress (RR: 2.3 [CI: 1.39–3.81]), necrotizing enterocolitis (RR: 5.54 [CI: 1.04–29.56]), and NICU admission (RR: 1.32 [CI: 1.13–1.55]). (2)(11)(13) The results of a systematic review of the frequency of complications observed in 39 cohorts of patients who underwent expectant management of severe preeclampsia remote from term are displayed in Table 3. (13) View this table: In this window In a new window Table 2. Timing of Delivery View this table: In this window In a new window Table 3. Complications of Expectant Management of Severe Preeclampsia Remote From Term When delivery is indicated, it is possible to achieve a vaginal delivery. However, this option becomes less likely with decreasing gestational age. When labor is induced at less than 28 weeks’ gestation, the cesarean delivery rate approaches 93% to 97%. In comparison, only 31% to 38% of women induced at 32 to 34 weeks’ gestation will require a cesarean delivery. (2) Previous SectionNext Section Maternal Complications Complications, especially in severe, early-onset disease, have been seen in every organ system. Preeclampsia is characterized by a constricted plasma volume compartment as well as by alterations in vasculature; thus, decreased arterial and venous compliance occurs, which can contribute to myocardial dysfunction, stroke, acute respiratory distress, coaguloapathy, renal or hepatic failure, and retinal injury. (14) In addition to eclampsia, other central nervous system complications include stroke and posterior reversible encephalopathy syndrome (PRES). PRES is characterized by vasogenic cerebral edema and infarctions in the subcortical white matter, predominantly in the parieto-occipital lobe. Clinical signs include headache, seizures, altered mental status, and hypertension. The edema associated with PRES seems to be due to endothelial damage and not a direct result of acutely elevated blood pressures. Evidence that PRES is caused by antiangiogenic factors is supported by the fact that it has also been seen in patients who have malignancies treated with antiangiogenic agents. (1) Figure 2 displays the classic magnetic resonance imaging findings of PRES. View larger version: In this page In a new window Download as PowerPoint Slide Figure 2. Classic magnetic resonance imaging findings of posterior reversible encephalopathy syndrome (PRES). Extensive areas of subcortical increased T2 signal with enhanced diffusivity involving bilateral posterior parietal and occipital white matter. Obstetric Triage and Emergency Care Protocols, Angelini & Fontaine, 2012, Courtesy of Springer Publishing Company, LLC. Cardiac abnormalities include asymptomatic left ventricular dysfunction and hypertrophy. In a study of women who had early-onset severe preeclampsia, 20% had severe hypertrophy, 52% had global diastolic dysfunction, and 26% had global systolic dysfunction. Although the women were usually asymptomatic at the time, epidemiologic studies have shown a high prevalence of essential hypertension and an increased cardiovascular risk status within a few years postpartum in what seems to be a dose-dependent relationship between preeclampsia and long-term morbidity. (14) Previous SectionNext Section Neonatal Complications Preeclampsia is the leading cause of iatrogenic premature delivery, and prematurity therefore leads to much of the observed neonatal morbidity. (15)(16) Several studies have compared infants born prematurely to healthy mothers versus those born to mothers with severe preeclampsia. Although some studies have shown no difference in outcomes, including respiratory distress syndrome, intraventricular hemorrhage, and periventricular leukomalacia, this is not a consistent finding. (17) In a study comparing the two populations, the incidence of a composite outcome (need for respiratory support, intraventricular hemorrhage, necrotizing enterocolitis, seizures, or neonatal mortality) was significantly higher in infants born after spontaneous labor to healthy mothers at 24 to 27 weeks and 6 days (100% vs 71%; P = .008). However, the opposite relationship was observed in infants 32 to 33 weeks and 6 days, suggesting the fetus receives some degree of protection when delivered very early but develops potential compromise when exposed to placental insufficiency for a prolonged period of time. (15) The reduced placental perfusion of preeclampsia also leads to neonatal complications, including growth restriction and fetal death. In the Magpie trial, the risk of fetal death in severe disease was 11.5%, twice as high as the rate of 5.4% seen in patients who had nonsevere disease. (12) Severe preeclampsia is the most common cause for intrauterine growth restriction in the nonanomalous infant. In a study of 307 infants born to mothers with preeclampsia compared with infants born to healthy mothers, birthweight was reduced 5% overall and 12% in early-onset disease. (18) In a separate review, the incidence of growth restriction in women who had severe preeclampsia remote from term was 28%, but after 32 weeks, this risk decreased to 9%, probably reflecting a more benign process. (15) The antiangiogenic state of the mother is also shared by the fetus, as cord blood VEFG and placental growth factor levels are decreased, whereas sFlt-1 levels are increased. This may contribute to the increased risk for bronchopulmonary dysplasia. An angiogenic state is required for normal pulmonary vascular development and airway branching, and adequate VEGF signaling is needed to maintain the alveolar structure of the lungs. A prospective cohort of 107 infants born between 23 and 32 weeks’ gestation showed an odds ratio of 2.96 for developing bronchopulmonary dysplasia if the mother had preeclampsia. (16)(19) Hematologic abnormalities may also be seen immediately after birth. The severity of dysfunction is proportional to the degree of growth restriction and placental dysfunction and not related to maternal hematologic abnormalities. Neutropenia is observed in 40% to 50% of neonates who are growth restricted and is potentially due to decreased production secondary to placental inhibitors. (15)(16) In a study of 520 hypertensive mothers, the rate of neonatal thrombocytopenia was 9.2% compared with 2.2% in controls. (20) However, term infants of hypertensive mothers were no more likely to be thrombocytopenic than control infants. This finding may be secondary to decreased production and microangiopathic sequestration of the platelets in the placental thrombi. These abnormalities are rarely severe and resolve in 1 to 3 days. (15)(16)(20) It has been hypothesized that an abnormal intrauterine environment causes long-term effects in adult life. Known as “the developmental origin of adult diseases,” this theory implies that chronic adult disease such as diabetes, hypertension, and cardiovascular disease have their origin in utero due to insults during critical periods of development. (21) A growing body of evidence in both laboratory and epidemiologic science supports this theory. In a study of 12-year-old children, those born to preeclamptic mothers had significantly higher systolic and diastolic blood pressure even after adjusting for weight and height. (22) In addition, after subdividing into children who were small for gestational age, children of mothers with preeclampsia who were small for gestational age had the highest levels of total cholesterol, low-density lipoprotein cholesterol, triglycerides, insulin, and plasma epinephrine. Another population-based study of over one million children showed an increased risk of endocrine, nutritional, and metabolic derangements in adolescence and early adulthood among the cohort exposed to preeclampsia in utero, risk factors that remained even after adjusting for differences in lifestyle. (23) The chronic placental insufficiency present in preeclampsia has the potential to influence fetal brain perfusion and lead to long-term effects on brain development and intelligence. Multiple studies have shown that children born to mothers who have preeclampsia may have various degrees of neurodevelopmental delay. (24)(25) In a study of 78 very low birthweight infants born before 32 weeks’ gestation, assessments at 2 years of corrected age (using the Bayley Scales of Infant Development), infants of mothers who had preeclampsia had significantly lower scores compared with infants born to healthy mothers. (25) In a retrospective study of 5,460 mothers with preeclampsia, a significant 1.46 increased risk of epilepsy was noted in their offspring compared with women who did not have preeclampsia. (26) This finding is supported by a separate study in the Danish population noting increased risks of epilepsy in the offspring of mothers with preeclampsia compared with offspring of healthy women. (27) Previous SectionNext Section Conclusions Preeclampsia, especially early-onset severe disease, is a complex disorder capable of leading to significant short- and long-term maternal and neonatal morbidity. Clinical manifestations of preeclampsia are a common end point for a number of complex pathophysiologic changes that seem to be a result of abnormal placentation. Because the only definitive therapy remains delivery, management of the disorder, especially when presenting in early gestation, continues to pose a challenge and focuses on minimizing significant maternal morbidity while achieving maximal benefit for the neonate. Infants born to mothers who have preeclampsia are at risk for both immediate and long-term health consequences of their abnormal uterine environment. American Board of Pediatrics Neonatal-Perinatal Content Specifications Know the normal morphologic development of the placenta. Know the effects on the fetus and/or newborn infant of mild preeclampsia and its management. Know the effects on the fetus and/or newborn infant of severe preeclampsia, including hemolysis, elevated liver enzymes, low platelet count syndrome, and its management. Previous SectionNext Section Footnotes Author Disclosure Drs McDonnold and Olson have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device. Abbreviations: CI; confidence interval PRES; posterior reversible encephalopathy syndrome RR; relative risk sFlt-1; soluble Fms-like tyrosine kinase-1 VEGF; vascular endothelial growth factor Copyright © 2013 by the American Academy of Pediatrics Previous Section References 1. 1.↵ 1. Maynard SE, 2. Karumanchi SA . Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31(1):33–46 CrossRefMedline 2. 2.↵ 1. Sibai BM ; Publications Committee, Society for Maternal-Fetal Medicine. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198 CrossRefMedline 3. 3.↵ 1. Zavalza-Gómez AB . Obesity and oxidative stress: a direct link to preeclampsia? Arch Gynecol Obstet. 2011;283(3):415–422 CrossRefMedline 4. 4.↵ 1. George EM, 2. Granger JP . Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24(9):964–969 CrossRefMedline 5. 5.↵ 1. 2. 3. 4. Silasi M, Cohen B, Karumanchi S, Rana S . Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin N Am. 2010;37(2):239–253 CrossRefMedline 6. 6.↵ 1. 2. 3. 4. 5. 6. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP . Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–H550 Abstract/FREE Full Text 7. 7.↵ 1. Redman C, 2. Sargent I . Preeclampsia, the placenta, and the maternal systemic inflammatory response – a review. Placenta. 2003;24(suppl A):S21–S27 Search Google Scholar 8. 8.↵ 1. Roberts J, 2. Funai E . Pregnancy related hypertension. In: Creasy RK, ed. Creasy and Resnik’s Maternal Fetal Medicine: Principles and Practice. 6th ed. Philadelphia, PA: Saunders Elsevier; 2009:651–688 Search Google Scholar 9. 9.↵ 1. 2. 3. 4. 5. 6. Spong CY, Mercer BM, D’alton M, Kilpatrick S, Blackwell S, Saade G . Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 pt 1):323–333 CrossRefMedline 10. 10.↵ 1. 2. 3. 4. Koopmans CM, Bijlenga D, Groen H, et al ; HYPITAT study group. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979–988 CrossRefMedlineWeb of Science 11. 11.↵ 1. Sibai B, 2. Barton J . Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196(6):514.e1–514.e9 Search Google Scholar 12. 12.↵ 1. 2. 3. 4. Altman D, Carroli G, Duley L, et al ; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890 CrossRefMedlineWeb of Science 13. 13.↵ 1. 2. 3. 4. 5. 6. Magee LA, Yong PJ, Espinosa V, Côté AM, Chen I, von Dadelszen P . Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy. 2009;28(3):312–347 CrossRefMedline 14. 14.↵ 1. Melchiorre K, 2. Thilaganathan B . Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23(6):440–447 CrossRefMedline 15. 15.↵ 1. Gruslin A, 2. Lemyre B . Pre-eclampsia: fetal assessment and neonatal outcomes. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):491–507 CrossRefMedline 16. 16.↵ 1. 2. 3. 4. Backes C, Markham K, Moorehead P, Cordero L, 5. Nankervis C, 6. Giannone P . Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365 Search Google Scholar 17. 17.↵ 1. 2. 3. 4. Kimberlin DF, Hauth JC, Owen J, et al . Indicated versus spontaneous preterm delivery: An evaluation of neonatal morbidity among infants weighing </=1000 grams at birth. Am J Obstet Gynecol. 1999;180(3 Pt 1):683–689 CrossRefMedlineWeb of Science 18. 18.↵ 1. 2. 3. 4. 5. Odegård RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R . Preeclampsia and fetal growth. Obstet Gynecol. 2000;96(6):950–955 CrossRefMedlineWeb of Science 19. 19.↵ 1. 2. 3. 4. Hansen AR, Barnés CM, Folkman J, McElrath TF . Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156(4):532–536 CrossRefMedlineWeb of Science 20. 20.↵ 1. Burrows RF, 2. Andrew M . Neonatal thrombocytopenia in the hypertensive disorders of pregnancy. Obstet Gynecol. 1990;76(2):234–238 MedlineWeb of Science 21. 21.↵ 1. 2. 3. 4. 5. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME . Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567 Abstract/FREE Full Text 22. 22.↵ 1. 2. 3. 4. 5. Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R . Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12year-old children born with maternal preeclampsia. J Clin Endocrinol Metab. 2003;88(3):1217–1222 Abstract/FREE Full Text 23. 23.↵ 1. 2. 3. 4. 5. 6. Wu C, Nohr E, Bech B, Vestergaard M, Catov J, Olsen J . Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201(3):269.e1–269.e10 Search Google Scholar 24. 24.↵ 1. 2. 3. 4. 5. Silveira RC, Procianoy RS, Koch MS, Benjamin AC, Schlindwein CF . Growth and neurodevelopment outcome of very low birth weight infants delivered by preeclamptic mothers. Acta Paediatr. 2007;96(12):1738–1742 CrossRefMedline 25. 25.↵ 1. 2. 3. 4. 5. Cheng SW, Chou HC, Tsou KI, Fang LJ, Tsao PN . Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum Dev. 2004;76(1):39–46 CrossRefMedlineWeb of Science 26. 26.↵ 1. Mann JR, 2. McDermott S . Maternal pre-eclampsia is associated with childhood epilepsy in South Carolina children insured by Medicaid. Epilepsy Behav. 2011;20(3):506–511 CrossRefMedline 27. 27.↵ 1. 2. 3. 4. Wu CS, Sun Y, Vestergaard M, et al . Preeclampsia and risk for epilepsy in offspring. Pediatrics. 2008;122(5):1072– 1078 Abstract/FREE Full Text Intrapartum Asphyxia, Neonatal Encephalopathy, Cerebral Palsy, and Obstetric Interventions in the Term and Near-Term Infant 1. Shannon M. Clark, MD, 2. Sanmaan K. Basraon, MD, and 3. Gary D.V. Hankins, MD + Author Affiliations 1. University of Texas Medical Branch, Galveston, TX. Next Section Abstract Intrapartum asphyxia (IA) as a cause of neonatal encephalopathy (NE) and cerebral palsy (CP) is a concern for obstetric providers due to the significant neonatal sequelae that ensue. CP is a nonprogressive static neuromuscular disorder appearing early after birth that occurs in 2 per 1,000 births. NE is a clinical syndrome of disturbed neurologic function in the first week after birth, and it occurs in 6 per 1,000 live births. Only ∼6% of all term infants diagnosed with CP have a history of NE, and without the development of NE, IA cannot be considered as the sole cause of CP. There are various preconceptional, antepartum, and intrapartum risk factors associated with CP. Obstetric interventions, including various modalities of fetal monitoring and cesarean delivery, have not led to improvement in outcomes or a reduction in the incidence of CP. The goal of this review was to discuss the association of IA with NE and CP in term and near-term infants, with a focus on the diagnosis and risk factors for IA and potential obstetric interventions. Previous SectionNext Section Objectives After completing this article, readers should be able to: 1. Differentiate between cerebral palsy, neonatal encephalopathy, and intrapartum asphyxia. 2. Define the criteria required to diagnose intrapartum asphyxia as a cause of moderate to severe neonatal encephalopathy. 3. Identify preconceptional, antepartum, and intrapartum risk factors for the development of neonatal encephalopathy and/or cerebral palsy. 4. Recognize potential obstetric interventions. Previous SectionNext Section Introduction In the majority of the cases of cerebral palsy (CP), the timing of insult is largely unknown, and an isolated intrapartum event causing asphyxia is rarely the cause of neurologic damage. It is prudent to understand the risk factors associated with the development of intrapartum asphyxia (IA) and the pathophysiology behind the development of neonatal encephalopathy (NE) and CP. Currently, there is a dearth of obstetric interventions that decrease the incidence of these disorders; thus, further research is needed to develop preventive strategies and targeted interventions aimed at improving neonatal outcomes. The goal of this review was to discuss the association of IA with NE and CP in term and near-term infants, with a focus on the diagnosis and risk factors for IA and potential obstetric interventions. Previous SectionNext Section The Connection Definition and Incidence CP is defined as a nonprogressive static neuromuscular disorder characterized by an abnormal control of movement or posture appearing early in life. (1) The onset occurs no later than age 1 year, and the definitive diagnosis is typically reserved until age 4 to 5 years. The prevalence of CP is ∼2 per 1,000 live births. Although term infants are at relatively low risk for CP, approximately one half of all births with CP are term and near-term infants (2) as term births constitute about 92% of all births. Whereas CP has many causes that may or may not be recognized at birth, NE is recognizable at birth. NE is a clinically defined syndrome of disturbed neurologic function manifested by difficulty with initiating and maintaining respirations, depression of tone and reflexes, altered level of consciousness, and often seizures in the first week after birth in the nearterm and term infant. (3)(4)(5)(6) Such acute neonatal neurologic dysfunction is the earliest and best indicator of neurologic injury and increased risk for later neurodevelopmental sequelae. (7) NE occurs in 1 to 6 per 1,000 live term births, (2) with 15% to 20% of affected newborns dying in the postnatal period and an additional 25% sustaining childhood disabilities. (8) IA is a known cause of NE. A key feature in the consideration of IA as a cause of CP in an individual infant is the concomitant presence of symptoms of moderate to severe NE. However, only 6% of all term infants diagnosed with CP have a history of NE; (9) therefore, the great majority of term CP cannot be considered to be the result of an intrapartum injury. (10) The reported incidence of IA in term or near-term infants is 1 to 8 neonates per 1,000 live term births, with 0.5 to 1.6 per 1,000 subsequently developing NE. (11) Of those with NE due to IA, between 10% and 60% will die, and ∼25% of the survivors will have long-term neurodevelopmental sequelae. (4) Other investigators (6)(7)(8)(9)(10)(11)(12) have reported that only 8% to 15% of term infants with NE, and even fewer with early neonatal seizures, (13) have evidence of asphyxia immediately before birth. Finally, IA as a cause of CP occurs in only a minority of cases and has been cited as low as 10% and as high as 20%. (14)(15) Timing of Injury Even though guidelines for diagnosing IA as a cause of NE have been established, determining if asphyxia was present before the onset of labor or developed during labor and delivery is difficult to ascertain. In addition, intrapartum adverse events could be the result of an antepartum predisposition or antepartum onset of brain injury or brain dysfunction that results in a negative response of the fetus to the stresses incurred during labor and delivery. (16)(17) As such, difficulties during the course of labor and delivery could be secondary to fetal compromise predating labor, and subsequent poor oxygenation or fetal hypotension during labor are merely contributors to the development of NE. (17) However, even if antenatal factors are identified, it is still difficult to prove that subsequent injury did not occur during the intrapartum period. The Western Australian case-control study by Badawi et al (4) in 1998 compared 164 term infants exhibiting moderate to severe NE (broadly defined) with 400 randomly selected controls. They found no evidence of intrapartum hypoxia in more than 70% of NE cases, and isolated IA accounted for only 4% of moderate to severe NE. Furthermore, they noted that most causes of NE were heterogeneous and most commenced in the antenatal period. These findings were in agreement with an international consensus statement, which noted that epidemiologic studies suggest that ∼90% of cases of CP have no history of IA. (18) In the remaining 10%, intrapartum signs compatible with damaging hypoxia may have had either antenatal or intrapartum origins. However, there are conflicting reports that suggest otherwise. In 2003, Cowan et al (19) used neonatal brain magnetic resonance imaging or postmortem examination in 351 term infants with NE, early seizures, or both to distinguish between lesions acquired antenatally and those that developed in the intrapartum and early postpartum period. They found that greater than 90% of term infants with NE, seizures, or both, but without specific syndromes or major congenital defects, had evidence of perinatally acquired insults, and there was a very low rate of established brain injury acquired before birth. However, their data could not exclude the possibility that antenatal factors might contribute to perinatal brain injury and, in addition to genetic predispositions to hypoxic-ischemic injury, may render the fetus more susceptible to the stresses of labor and delivery. Previous SectionNext Section Clinical Criteria for Diagnosing Intrapartum Asphyxia Pathophysiology of Intrapartum Asphyxia The diagnosis of IA (fetal hypoxia and/or ischemia) includes impaired respiratory gas exchange and development of fetal metabolic acidosis (20) during labor and delivery with oxygen deprivation in fetal brain either through hypoxia and/or ischemia. Hypoxemia results from diminished oxygen in the fetal blood supply, and cerebral ischemia occurs due to impaired blood supply to the fetal brain, with the latter resulting in deprivation of glucose, which further contributes to the development of neuronal injury. (21) When an intrapartum insult occurs as a result of various events, the placental blood flow and gas exchange is impaired, leading to a cycle of ischemia and reperfusion of the fetal brain that results in necrosis and cell death. (22)(23) After such insult, the fetal cardiac output is redistributed, with decreased blood flow to the lungs, kidney, and intestine with preservation of circulation to the brain, heart, and adrenals. (24) Of utmost importance is the maintenance of the integrity of the central nervous system (CNS), especially the brain, during the compensatory phase of an asphyxial exposure that is accomplished by a combination of increased cerebral blood flow and oxygen extraction. (24) Damage is greatest when the asphyxial exposure persists and cardiovascular decompensation occurs. A severe metabolic acidosis then develops, and the combination of asphyxia and ischemia due to hypotension and hypoperfusion results in a decrease in cerebral oxygen consumption and ultimately brain injury and end-organ damage. (24) A fetus can usually compensate and recover after an asphyxial exposure with correction of respiratory acidosis. (25) However, recurrent, intermittent, or continuous asphyxial insults causing metabolic acidosis for a prolonged period of time can result in varying degrees of brain injury depending on the gestational age of the fetus. Ultimately, these events can lead to short-term sequelae, as represented by NE, or long-term sequelae, as seen with CP. Clinical Diagnosis of Intrapartum Asphyxia Currently, the standard for defining an acute intrapartum hypoxic-ischemic event as sufficient to cause moderate to severe NE in term and near-term neonates that subsequently results in CP uses the four essential criteria put forth by the American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force on Neonatal Encephalopathy and Cerebral Palsy (Table 1). (26) If all of these criteria are met, it is likely that the pathology causing CP occurred during labor. The task force also presented five criteria that suggest an intrapartum timing of a hypoxic event within 48 hours of delivery when present collectively but are nonspecific to asphyxial insults. These criteria are weakly associated with an acute IA event, with the exception of the first one (a sentinel or hypoxic event occurring immediately before or after the onset of labor). (3) View this table: In this window In a new window Table 1. Criteria for Defining an Acute Intrapartum Hypoxic-Ischemic Event as a Cause of CP (26) Even when all four essential criteria are met, the timing of the insult cannot be definitely determined. Hypoxia could be intermittent, chronic, or acute during labor in a previously healthy fetus. However, if an ischemic cerebral injury occurred in the intrapartum period, results of the neurologic examination of the neonate will be abnormal within the first 24 hours of birth. Abnormalities can be observed in the following: 1) cortical function (lethargy, stupor, coma with or without seizures); 2) brainstem function (pupillary and cranial nerve abnormalities); 3) tone (hypotonia); and 4) reflexes (absent, hyporeflexia). (7(21) If the NE that develops after an IA event is severe enough to cause CP, it is of the spastic quadriplegic or dyskinetic type. (3)(27)(28) Unilateral brain lesions, hemiparetic CP, (29)(30) hemiplegic CP, spastic diplegia, and ataxia have not been associated with acute IA. (30) Finally, any progressive neurologic disability is not CP and thus not a result of an acute IA event. (3) When considering fetal tracings, abnormal patterns most frequently found to be associated with the development of CP include multiple late decelerations and decreased beat-to-beat variability. (3) Of note, the presence of such tracings may be the first sign of a pre-existing fetal neurologic abnormality, (31) severe antenatal neurologic injury, (32)(33) or an acute intrapartum injury. However, there is a high false-positive rate when predicting CP. (34) Nelson et al (34) found that intrapartum fetal heart rate tracing abnormalities as a marker for IA had poor predictive value for the development of NE; the presence of multiple late decelerations and/or persistent decreased beat-tobeat variability had a false-positive predictive rate for subsequent development of CP of 99.8%. Similarly, it is known that 1- or 5-minute Apgar scores alone are poor predictors of long-term neurologic outcome, with 75% of children with CP obtaining normal Apgar scores at birth. (35) Finally, extremely low Apgar scores at 15 and 20 minutes only have been shown to strongly correlate with subsequent neurologic dysfunction. (3) The best indicator for IA is metabolic acidosis (pH <7 and base deficit ≥12 mmol/L) in umbilical arterial blood at the time of delivery. (18)(36) This finding allows for an accurate diagnosis of asphyxia via umbilical cord blood gas and acid base assessment. (24) It is recommended that both arterial and venous cord blood be obtained because arterial blood reflects fetal status more directly and venous blood reflects whether the uteroplacental oxygen exchange is optimal; (37) however, this testing is not always feasible. If there is significant metabolic acidosis at the time of sampling, it is likely that IA has occurred. However, it does not indicate the duration of exposure or whether it was continuous or intermittent. (24) The diagnosis of IA is based on the presence of metabolic acidosis; the severity of the asphyxia is based on the degree of NE and the presence of other organ system complications. (24) Multisystem organ involvement, once thought to be a requirement for the diagnosis of IA, was included on the list of nonspecific criteria for hypoxic-ischemic encephalopathy (HIE) by the American College of Obstetricians and Gynecologists/American Academy of Pediatrics Task Force. (26) As previously mentioned, during an IA event, an attempt is made to preserve perfusion to the vital organs by shunting blood away from other organ systems. (11) As a result, elevation in liver enzyme levels, impaired renal function and acute tubular necrosis, and heart injury may be observed in the neonate. Laboratory assessment should occur as soon as possible after delivery if IA is believed to be present, and followed over the next several days to weeks because some markers do not immediately appear as abnormal. In a 2002 study by Hankins et al, (38) 46 cases of acute peripartum asphyxia sufficient to result in the diagnosis of NE were identified through a prospectively maintained database. Using criteria to define an acute IA event that are slightly different from what is used today, the authors identified how often various organ systems reflected injury patterns by using commonly available laboratory tests and/or imaging technologies. They included patients with an obvious acute intrapartum event of recent onset, such as a placental abruption, umbilical cord prolapse, or deterioration in a previously normal fetal heart rate pattern in gestations greater than 32 weeks. They found that in cases with clinical CNS injury resulting in encephalopathy, 49% had abnormal results on electroencephalogram and 40% had imaging studies that were diagnostic of acute injury. In addition, liver injury, based on elevated transaminase levels, occurred in 80%; heart injury, as defined by pressor or volume support beyond 2 hours after birth or elevated cardiac enzyme levels, occurred in 78%; and renal injury, defined by an elevation of serum creatinine to greater than 1.0 mg/dL, persistent hematuria, persistent proteinuria, or clinical oliguria, occurred in 72%. Finally, when combining results of laboratory and imaging studies, involvement of the renal, hepatic, CNS, and cardiac systems was observed in greater than 70% of cases. Hankins et al concluded that multiple organs suffer damage during an acute IA event sufficient to result in NE, and absence of injury does not correlate with the diagnosis of IA. Previous SectionNext Section Risk Factors Preconceptional and Antepartum Risk Factors There is a higher incidence of maternal illness, antenatal complications, and adverse social factors in infants with NE, seizures, or both, (2)(3)(5)(30)(39)(40)(41)(42)(43)(44) most occurring occur well before birth, which must be excluded before the diagnosis of IA is made. (19)(26) As previously discussed, the presence of antepartum risk factors may render the fetus more susceptible to the stresses of labor and delivery, and further increase the risk of IA. In this scenario, it is impossible to determine whether the antepartum risk factor(s) or an intrapartum insult played the key role in the development of NE and/or CP. Conversely, the presence of antepartum risk factors does not mean that an acute intrapartum event cannot occur. In addition to intrapartum insults, Badawi et al, (5) in their population-based unmatched case-control study of term infants, found various preconceptional and antenatal factors that increase the risk of NE and/or CP as summarized in Tables 2 and 3. Various social factors, family and personal history, and infertility treatment were strongly associated with risk of development of NE and/or CP. In addition, various antenatal factors, most importantly maternal thyroid disease, severe preeclampsia, and intrauterine growth restriction (less than the third percentile for birthweight), had a strong association with NE. They concluded that there are numerous causes of NE, many of which start before labor and delivery. In 2011, Maisonneuve et al (45) identified risk factors of severe acidosis in a case-control study of term pregnancies with severe neonatal acidosis (umbilical artery pH < 7.0) and found severe acidosis in 0.63% of 39,321 live term births. They did not use all criteria for the diagnosis of IA for the purposes of this study. Maternal age greater than 35 years, previous neonatal death and previous cesarean delivery (CD), were independent risk factors for severe neonatal acidosis (Tables 2 and 3). View this table: In this window In a new window Table 2. Preconceptional Risk Factors for NE and/or CP View this table: In this window In a new window Table 3. Antepartum Risk Factors for NE and/or CP Chorioamnionitis (intrapartum maternal and/or fetal tachycardia and maternal temperature elevation) increase the risk of NE and CP. (2) Although chorioamnionitis is readily identifiable during the course of labor and delivery, other antepartum infections are variable in their sequelae and ease of diagnosis. It is well known that rubella and cytomegalovirus are viral teratogens; however, there are other viruses that may play a role in fetal neurologic damage in the antepartum period. (46) In addition, maternal hyperthermia, inflammatory mediators, and other pathophysiologic sequelae observed with any maternal infection may contribute to the development of IA and NE. (47) In the event that antenatal or intrapartum exposure to infection occurs, neonates should be evaluated by using proper laboratory assessments and examination, and, ideally, the placenta should be sent for pathologic evaluation. Intrapartum Risk Factors A sentinel event (SE) is an acute intrapartum pathologic event that causes neurologic damage to a previously intact fetus through compromised blood or oxygen supply. (3) Although the task force considers it as a criterion suggestive of an IA event as a cause of NE, an SE is more strongly associated with acute IA than other criteria. (26) Examples of SEs and intrapartum risk factors are shown in Table 4. (2)(3) View this table: In this window In a new window Table 4. Intrapartum Risk Factors and Sentinal Events (*) for NE and/or CP A retrospective population-based study by Gilbert et al (48) in 2010 examined adverse intrapartum events in children with spastic quadriplegic or dyskinetic type CP. These events included abruption, uterine rupture, fetal distress, birth trauma, prolapsed cord, and mild to severe birth asphyxia. The frequency of CP within the study population was 1.4 per 1,000 deliveries. Overall, 31.3% of these children had one or more of the six adverse intrapartum events compared with 12.9% of controls. Fifty-nine percent (4,274 of 7,242) of children identified with CP in this study were term births; 28.3% had one or more adverse events compared with 12.7% in controls. The authors noted that these findings could show that birth-related events are a more significant cause of the development of CP than previously thought. However, overall, the majority of children who had CP did not have an adverse intrapartum event related to their development of CP. In a 2012 retrospective double cohort study of three groups of infants at greater than 35 weeks’ gestation exposed to different risk factors for IA, Martinez-Biarge et al (49) examined perinatal morbidity and the rate of HIE in infants exposed to intrapartum SE. These groups included the following: (1) infants with an intrapartum SE; (2) infants delivered by emergency CD or operative vaginal delivery due to abnormal fetal heart rate tracing; and (3) infants delivered by elective CD before the onset of labor. SE included uterine rupture, placental abruption, cord prolapse, and amniotic fluid embolism. Diagnosis of HIE was made when the infant met the criteria for neonatal depression/cord arterial pH ≤7.00 or Apgar score ≤3 at 1 minute and/or ≤5 at 5 minutes or need for advanced resuscitation; and NE as presented by Leviton and Nelson. (6) They found that perinatal mortality was 6% in the SE group and 0.3% in the nonreassuring fetal status group (relative risk [RR]: 2.4 [95% confidence interval (CI): 1.95–2.94]) with perinatal morbidity increased two to six times in infants exposed to SE. The incidence of HIE was 10% in the SE group compared with 2.5% in the nonreassuring fetal status group (RR: 1.93 [95% CI: 1.49–2.52]). When considering SE, uterine rupture was associated with the highest incidence of HIE (32%), followed by placental abruption (11%). Finally, no infant in the elective cesarean group died, had perinatal morbidity, or developed encephalopathy. The authors concluded that intrapartum SE are a significant cause of perinatal morbidity and the development of HIE. (49) The 1998 Australian study of Badawi et al (4) also examined various intrapartum predictors for NE in term infants (Table 4). They found that the prevalence of moderate or severe NE was 3.8 per 1,000 term live births with a neonatal mortality rate of 9.1%. When considering risk factors, 69% of case infants had only antepartum risk factors for NE, 24% had antepartum and intrapartum factors, 5% had only intrapartum factors, and 2% had no identifiable risk factors. They concluded that IA accounts for a small proportion of cases of NE, and elective CD has an inverse association with NE. Previous SectionNext Section Obstetric Interventions Electronic Fetal Monitoring For the term and near-term infant, the most obvious tool that the obstetrician can use to identify fetuses at risk for IA and decrease the risk of intrapartum fetal death or neonatal seizures is electronic fetal monitoring (EFM) during labor and delivery. (50) Although some studies report that EFM correlates with the onset of metabolic acidosis and subsequent neurologic injury, (50)(51) there is no consensus on this subject, and investigators disagree on what is the most ominous fetal heart rate pattern that signifies potential metabolic acidosis. In general, prolonged decreased variability with repetitive, prolonged late or variable decelerations and sustained bradycardia are all potential signs of impending neonatal metabolic acidosis. In general, however, the predictive power of EFM for the development of NE and CP is low, (25) and to date, the use of EFM has not decreased the incidence of CP. (52) This is likely due to the fact that most cases of CP are a result of events that occurred before the onset of labor, and a very small percentage of cases are a result of IA. (3)(5) Overall, EFM has led to an increase in obstetric interventions in the form of cesarean and operative vaginal deliveries, especially during active labor. (53) This affords the potential for increased complications for both the mother and fetus. Despite this risk, EFM is the best tool obstetricians can use in labor in an effort to identify fetal metabolic acidosis. ST waveform analysis includes the addition of the fetal electrocardiogram to standard cardiotocography for intrapartum fetal monitoring in an attempt to reduce neonatal and fetal asphyxia. (54)(55) A meta-analysis of randomized controlled trials by Becker et al (56) in 2012 compared the effects on ST waveform analysis with standard continuous cardiotocography in singleton pregnancies in cephalic presentation at ≥34 weeks’ gestation. They evaluated various abnormalities in metabolic acidosis, umbilical cord pH, Apgar scores, admittance to the NICU, need for intubation, presence of HIE, perinatal death, operative delivery, and number of fetal blood samplings. They found that ST waveform analysis did not reduce the occurrence of metabolic acidosis (RR: 0.72 [95% CI: 0.43–1.19]). However, ST waveform analysis did significantly reduce the incidence of additional fetal blood sampling (RR: 0.59 [95% CI: 0.44–0.79]), operative vaginal deliveries (RR: 0.88 [95% CI: 0.80–0.97]), and total operative deliveries (RR: 0.94 [95% CI: 0.89–0.99]). (56) Although fetal blood sampling is not standard practice in most institutions, continuous cardiotocography for intrapartum fetal monitoring is still used. Trials are underway to determine if ST waveform analysis is effective in reducing the occurrence of neonatal metabolic acidosis. Cesarean Delivery CD has well-known surgical risk to the mother, especially with subsequent repeat CD. When considering the term infant and CD, the risk of respiratory complications is greater, especially in the case of elective CD in the absence of labor. There is evidence that CD for the breech fetus reduces perinatal mortality, neonatal mortality, and serious neonatal morbidity with a planned CD. (57) Although it seems reasonable that elective CD may decrease the incidence of IA, any benefit may indeed be due to avoidance of certain intrapartum risk factors rather than bypassing the potential for adverse intrapartum events. (4) Currently, there is no recommendation for the performance of elective CD for the prevention of IA and the subsequent development of NE and CP. Other Interventions One of the most obvious interventions obstetricians have is antenatal screening for the detection of risk factors for adverse antepartum and intrapartum outcomes. (25) Early detection allows earlier intervention and informed decision-making regarding mode and timing of delivery. Identification of maternal and fetal disease is of utmost importance. When considering labor and delivery, there are several interventions that obstetricians use when faced with a nonreassuring fetal heart tracing. These interventions include maternal oxygen supplementation and position change, tocolytic agents (ie, β2adrenergic agonist), and amnioinfusion. In some of these cases, these measures allow resuscitation before the decision is made to proceed with CD. American Board of Pediatrics Neonatal–Perinatal Content Specifications Know the clinical features, diagnosis, and management of perinatal hypoxicischemic encephalopathy. Differentiate asphyxia from other causes of depression at birth, including drug effects and hypovolemia. Understand the significance, limitations, and causes of low Apgar scores. Know the interpretation of fetal scalp and umbilical cord blood gas and pH values. Know the approximate risk of cerebral palsy in very low birthweight, moderately low birthweight, and normal birthweight infants. Know the relationship between Apgar scores and later development of cerebral palsy in preterm and term infants. Know the prenatal, perinatal, and neonatal risk factors for the development of cerebral palsy. Know that the majority of children with cerebral palsy have no identifiable cause. Previous SectionNext Section Footnotes Author Disclosure Drs Clark, Basraon, and Hankins have disclosed no financial relationships relevant to this article. This commentary does contain a discussion of an unapproved/investigative use of a commercial product/device. Abbreviations: CD; cesarean delivery CNS; central nervous system CP; cerebral palsy EFM; electronic fetal monitoring HIE; hypoxic-ischemic encephalopathy IA; intrapartum asphyxia NE; neonatal encephalopathy SE; sentinel event Copyright © 2013 by the American Academy of Pediatrics Previous Section References 1. 1.↵ 1. 2. 3. 4. Aisen ML, Kerkovich D, Mast J, et al . Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852 CrossRefMedline 2. 2.↵ 1. Shankaran S . Prevention, diagnosis, and treatment of cerebral palsy in near-term and term infants. Clin Obstet Gynecol. 2008;51(4):829–839 CrossRefMedline 3. 3.↵ 1. Hankins GD, 2. Speer M . Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol. 2003;102(3):628–636 CrossRefMedlineWeb of Science 4. 4.↵ 1. 2. 3. 4. Badawi N, Kurinczuk JJ, Keogh JM, et al . Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317(7172):1554–1558 Abstract/FREE Full Text 5. 5.↵ 1. 2. 3. 4. Badawi N, Kurinczuk JJ, Keogh JM, et al . Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317(7172):1549–1553 Abstract/FREE Full Text 6. 6.↵ 1. Leviton A, 2. Nelson KB . Problems with definitions and classifications of newborn encephalopathy. Pediatr Neurol. 1992;8(2):85–90 CrossRefMedlineWeb of Science 7. 7.↵ 1. Sarnat HB, 2. Sarnat MS . Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705 CrossRefMedlineWeb of Science 8. 8.↵ 1. 2. 3. 4. Badawi N, Felix JF, Kurinczuk JJ, et al . Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47(5):293–298 CrossRefMedlineWeb of Science 9. 9.↵ 1. Nelson KB . The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev. 2002;8(3):146–150 CrossRefMedlineWeb of Science 10. 10.↵ 1. Johnson SL, 2. Blair E, 3. Stanley FJ . Obstetric malpractice litigation and cerebral palsy in term infants. J Forensic Leg Med. 2011;18(3):97–100 CrossRefMedline 11. 11.↵ 1. Flidel-Rimon O, 2. Shinwell ES . Neonatal aspects of the relationship between intrapartum events and cerebral palsy. Clin Perinatol. 2007;34(3):439–449 CrossRefMedline 12. 12.↵ 1. Blair E, 2. Stanley FJ . Intrapartum asphyxia: a rare cause of cerebral palsy. J Pediatr. 1988;112(4):515–519 CrossRefMedlineWeb of Science 13. 13.↵ 1. 2. 3. 4. 5. 6. Mercuri E, Cowan F, Rutherford M, Acolet D, Pennock J, Dubowitz L . Ischaemic and haemorrhagic brain lesions in newborns with seizures and normal Apgar scores. Arch Dis Child Fetal Neonatal Ed. 1995;73(2):F67–F74 Abstract/FREE Full Text 14. 14.↵ 1. Shevell MI, 2. Majnemer A, 3. Morin I . Etiologic yield of cerebral palsy: a contemporary case series. Pediatr Neurol. 2003;28(5):352–359 CrossRefMedlineWeb of Science 15. 15.↵ 1. 2. 3. 4. Yudkin PL, Johnson A, Clover LM, Murphy KW . Assessing the contribution of birth asphyxia to cerebral palsy in term singletons. Paediatr Perinat Epidemiol. 1995;9(2):156–170 MedlineWeb of Science 16. 16.↵ 1. 2. 3. 4. 5. 6. Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA, Karchmar EJ . Motor and cognitive deficits after intrapartum asphyxia in the mature fetus. Am J Obstet Gynecol. 1988;158(2):356–361 MedlineWeb of Science 17. 17.↵ 1. de Vries LS, 2. Cowan FM . Evolving understanding of hypoxic-ischemic encephalopathy in the term infant. Semin Pediatr Neurol. 2009;16(4):216–225 CrossRefMedline 18. 18.↵ 1. MacLennan A . A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999;319(7216):1054– 1059 FREE Full Text 19. 19.↵ 1. 2. 3. 4. Cowan F, Rutherford M, Groenendaal F, et al . Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361(9359):736–742 CrossRefMedlineWeb of Science 20. 20.↵ 1. Shevell MI . The “Bermuda triangle” of neonatal neurology: cerebral palsy, neonatal encephalopathy, and intrapartum asphyxia. Semin Pediatr Neurol. 2004;11(1):24–30 CrossRefMedline 21. 21.↵ 1. Volpe JJ . Neurology of the Newborn. 4th ed. Philadelphia, PA: WB Saunders Company; 2001 Search Google Scholar 22. 22.↵ 1. Perlman JM . Intrapartum hypoxic-ischemic cerebral injury and subsequent cerebral palsy: medicolegal issues. Pediatrics. 1997;99(6):851–859 FREE Full Text 23. 23.↵ 1. 2. 3. 4. Lorek A, Takei Y, Cady EB, et al . Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36(6):699–706 MedlineWeb of Science 24. 24.↵ 1. Low JA . Determining the contribution of asphyxia to brain damage in the neonate. J Obstet Gynaecol Res. 2004;30(4):276–286 CrossRefMedlineWeb of Science 25. 25.↵ 1. Kumar S, 2. Paterson-Brown S . Obstetric aspects of hypoxic ischemic encephalopathy. Early Hum Dev. 2010;86(6):339–344 CrossRefMedlineWeb of Science 26. 26.↵ American College of Obstetricians and Gynecologists. The American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy and Cerebral Palsy, the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington, DC: the American College of Obstetricians and Gynecologists; 2003 27. 27.↵ 1. Rosenbloom L . Dyskinetic cerebral palsy and birth asphyxia. Dev Med Child Neurol. 1994;36(4):285–289 Medline 28. 28.↵ 1. Stanley FJ, 2. 3. 4. 5. Blair E, Hockey A, Petterson B, Watson L . Spastic quadriplegia in Western Australia: a genetic epidemiological study. I: Case population and perinatal risk factors. Dev Med Child Neurol. 1993;35(3):191–201 MedlineWeb of Science 29. 29.↵ 1. Michaelis R, 2. Rooschüz B, 3. Dopper R . Prenatal origin of congenital spastic hemiparesis. Early Hum Dev. 1980;4(3):243–255 CrossRefMedline 30. 30.↵ 1. Nelson KB, 2. Grether JK . Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179(2):507–513 CrossRefMedlineWeb of Science 31. 31.↵ 1. 2. 3. 4. 5. 6. Adamson SJ, Alessandri LM, Badawi N, Burton PR, Pemberton PJ, Stanley F . Predictors of neonatal encephalopathy in full-term infants. BMJ. 1995;311(7005):598–602 Abstract/FREE Full Text 32. 32.↵ 1. Phelan JP, 2. Ahn MO . Perinatal observations in forty-eight neurologically impaired term infants. Am J Obstet Gynecol. 1994;171(2):424–431 MedlineWeb of Science 33. 33.↵ 1. Schifrin BS, 2. Hamilton-Rubinstein T, 3. Shields JR . Fetal heart rate patterns and the timing of fetal injury. J Perinatol. 1994;14(3):174–181 Medline 34. 34.↵ 1. 2. 3. 4. Nelson KB, Dambrosia JM, Ting TY, Grether JK . Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–618 CrossRefMedlineWeb of Science 35. 35.↵ 1. Nelson KB, 2. Ellenberg JH . Apgar scores as predictors of chronic neurologic disability. Pediatrics. 1981;68(1):36–44 Abstract/FREE Full Text 36. 36.↵ American College of Obstetricians and Gynecologists. ACOG committee opinion. Use and abuse of the Apgar score. Number 174-July 1996 (replaces No. 49, November 1986). Committee on Obstetric Practice and American Academy of Pediatrics: Committee on Fetus and Newborn. Int J Gynaecol Obstet. 1996;54(3):303–305 CrossRefMedline 37. 37.↵ 1. Muraskas JK, 2. Morrison JC . A proposed evidence-based neonatal work-up to confirm or refute allegations of intrapartum asphyxia. Obstet Gynecol. 2010;116(2 pt 1):261–268 CrossRefMedline 38. 38.↵ 1. 2. 3. 4. 5. 6. Hankins GD, Koen S, Gei AF, Lopez SM, Van Hook JW, Anderson GD . Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol. 2002;99(5 pt 1):688–691 CrossRefMedlineWeb of Science 39. 39.↵ 1. Blair E, 2. Stanley F . Intrauterine growth and spastic cerebral palsy. I. Association with birth weight for gestational age. Am J Obstet Gynecol. 1990;162(1):229–237 MedlineWeb of Science 40. 40.↵ 1. Foley J . Birth-weight ratio and cerebral palsy. Early Hum Dev. 1995;40(2):145–156 CrossRefMedline 41. 41.↵ 1. Keogh JM, 2. Badawi N . The origins of cerebral palsy. Curr Opin Neurol. 2006;19(2):129–134 MedlineWeb of Science 42. 42.↵ 1. Nelson KB, 2. Ellenberg JH . Antecedents of cerebral palsy. I. Univariate analysis of risks. Am J Dis Child. 1985;139(10):1031–1038 CrossRefMedline 43. 43.↵ 1. Nuñez JL, 2. McCarthy MM . Sex differences and hormonal effects in a model of preterm infant brain injury. Ann N Y Acad Sci. 2003;1008:281–284 CrossRefMedlineWeb of Science 44. 44.↵ 1. Uvebrant P, 2. Hagberg G . Intrauterine growth in children with cerebral palsy. Acta Paediatr. 1992;81(5):407–412 MedlineWeb of Science 45. 45.↵ 1. 2. 3. 4. Maisonneuve E, Audibert F, Guilbaud L, et al . Risk factors for severe neonatal acidosis. Obstet Gynecol. 2011;118(4):818– 823 CrossRefMedline 46. 46.↵ 1. Halperin LR, 2. Wilroy RS Jr . Maternal hyperthermia and neural-tube defects. Lancet. 1978;2(8082):212– 213 Medline 47. 47.↵ 1. Adlinolfi M . Infectious diseases in pregnancy, cytokines and neurological impairment: an hypothesis. Dev Med Child Neurol. 1993;35(6):549–553 MedlineWeb of Science 48. 48.↵ 1. 2. 3. 4. 5. Gilbert WM, Jacoby BN, Xing G, Danielsen B, Smith LH . Adverse obstetric events are associated with significant risk of cerebral palsy. Am J Obstet Gynecol. 2010;203(4):328.e1–328.e 5 Search Google Scholar 49. 49.↵ 1. 2. 3. 4. 5. Martinez-Biarge M, Madero R, Gonzalez A, Quero J, Garcia-Alix A . Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am J Obstet Gynecol. 2012;206(2):148.e1– 148.e7 Medline 50. 50.↵ American College of Obstetricians and Gynecologists. Intrapartum Fetal Heart Rate Monitoring: Nomenclature, Interpretation, and General Management Principles. Washington, DC: the American College of Obstetricians and Gynecologists; 2009. ACOG Practice Bulletin Number 106 51. 51.↵ 1. Williams KP, 2. Galerneau F . Intrapartum fetal heart rate patterns in the prediction of neonatal acidemia. Am J Obstet Gynecol. 2003;188(3):820–823 CrossRefMedlineWeb of Science 52. 52.↵ 1. Clark SM, 2. Ghulmiyyah LM, 3. Hankins GD . Antenatal antecedents and the impact of obstetric care in the etiology of cerebral palsy. Clin Obstet Gynecol. 2008;51(4):775–786 CrossRefMedlineWeb of Science 53. 53.↵ 1. Nelson KB, 2. Chang T . Is cerebral palsy preventable? Curr Opin Neurol. 2008;21(2):129–135 CrossRefMedlineWeb of Science 54. 54.↵ 1. 2. 3. 4. Amer-Wåhlin I, Hellsten C, Norén H, et al . Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet. 2001;358(9281):534–538 CrossRefMedlineWeb of Science 55. 55.↵ 1. 2. 3. 4. 5. Ojala K, Vääräsmäki M, Mäkikallio K, Valkama M, Tekay A . A comparison of intrapartum automated fetal electrocardiography and conventional cardiotocography—a randomised controlled study. BJOG. 2006;113(4):419–423 CrossRefMedline 56. 56.↵ 1. 2. 3. 4. Becker JH, Bax L, Amer-Wåhlin I, et al . ST analysis of the fetal electrocardiogram in intrapartum fetal monitoring: a meta-analysis. Obstet Gynecol. 2012;119(1):145–154 CrossRefMedline 57. 57.↵ 1. 2. 3. 4. 5. 6. Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR ; Term Breech Trial Collaborative Group. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet. 2000;356(9239):1375–1383 CrossRefMedlineWeb of Science