High Iron Calcines Treatment in Electrolytic Zinc Plant of
advertisement

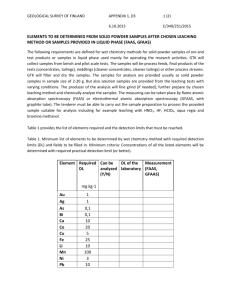
High Iron Calcines Treatment in Electrolytic Zinc Plant of Metallurgical Met-Mex Peñoles , S.A de C.V Claudio Rocha Rosales Metalurgica ,Met-Mex Peñoles S.A. C.V. Electrolytic Zinc Plant Boulevard Laguna 3200 Poniente, Colonia Metalurgica Torreon, Coahuila, Mexico claudio_rocha@penoles.com.mx ABSTRACT Peñoles is a mining group with integrated operations in the smelting and refining of non-ferrous metals, and is also a chemicals producer. Peñoles is the world’s foremost producer of refined silver, metallic bismuth and sodium sulphate and is the leading Latin American producer of refined gold, lead and zinc. The Met-Mex Peñoles Zinc Plant commenced operations in 1973 with an annual production capacity of 105,000 t/y. Year after year the production has been increased, and at present, the production capacity is 240,000 t/y. In this paper, the leaching process,particularly the operation of the jarosite circuit, is reviewed ,specifying process improvements to treat high iron calcines . INTRODUCTION The purpose of the leaching process is to recover the metals, mainly zinc, through a series of extraction stages with sulphuric acid solutions, which progressively increase the zinc recovery. After the series of acidification stages,the solution, which is rich in iron, is sent to the jarosite circuit to separate the zinc from the iron [1]. The first step of the leaching process is the neutral leach circuit which produces a zinc sulphate solution that is sent to the purification section for the removal of impurities such as cadmium, cobalt and copper, after this solution is sent to the tankhouse to feed the electrolytic cells. REVIEW OF THE LEACHING PROCESS Leaching Process The Leaching Process consists of the following stages, which are presented in Figure 1. • Neutral Leaching • Fourth Purification • Acid Leaching • Hot Acid Leaching • Jarosite Formation • Jarosite Acid Washing • Sulphates Purge • Lead–Silver Residue Filtration • Jarosite Filtration • Jarosite Neutralization LOW SILVER CALCINE LEACHING HIGH SILVER CALCINE LEACHING spent calcine MnO2 spent Neutral Leaching FourthPurifica tion ZnSO4 Neutral Leaching Neutral solution Neutral Thickener calcine Fourth Purification Neutral solution NH3 to Purification calcine to Purification Neutral Thickener Jarosite Acid Leaching Acid Leaching Jarosite Thickener Acid Thickener Acid Thickener spent H2SO4 Acid Wash of Jarosite spent Hot Leaching spent Hot Leaching H2SO4 Hot Thickener filtrate Acid Wash Thickener Water Wash Thickener Purge Sulphate solids PRESS FILTERS TRAY FILTERS filtrate filtrate solids Lead-Silver Cake to Lead Plant Hot Thickener Ca(OH)2 Spent solids PRESS FILTERS Jarosite Cake Hard Water to Water Treatment CaCO3 + Ca(OH)2 Neutralization Jarosite Neutralized Jarosite to Container Figure 1 – Leaching circuit Flow Diagram filtrate filtrate Lead-Silver Cake to Lead Plant solids solids Neutral Leaching The purpose of this stage is to leach most of the zinc present as ZnO in the calcine, and the zinc extraction is in the order of 85%. In this circuit, there is a reaction tank in which the acidity is controlled to a pH 1 to 1.5 . The attack acid is composed of spent electrolyte, manganese acid solution from the cellhouse and the zinc solution recovered from the purification section. Fourth Purification The purpose of this circuit is to integrate the zinc that was recovered in the residue treatment stages, because it is a solution with an average acidity of 15 g/L and an average iron concentration ranging from 4 to 8 g/L. This solution is used to leach calcine and simultaneously hydrolyze the iron and precipitate impurities like arsenic, antimony,indium, selenium and tellurium. In this stage,there is a reactor tank controlled to a pH of 4.0 to 4.5 . Acid Leaching The purpose of this operation is to leach the remaining zinc, present as ZnO, that was not leached in the previous stages to achieve a combined extraction in the order of 90%. There are three reactors where electrolyte is added to achieve a high zinc extraction. The free acidity in the first tank ranges from 15 to 25 g/L . Hot Acid Leaching The purpose of this circuit is to leach the zinc ferrites (ZnO.Fe2O3), and in this way, to increase the accumulated zinc extraction to 98%. In addition, a leadsilver residue is generated that is filtered and sent to the adjacent lead smelter to recover the lead and silver values. There are 10 reactor tanks, where sulphuric acid and spent electrolyte are added to maintain a free acidity in the first tank from 150 to 170 g/L; steam is added to the tanks to maintain a temperature of 85°C. Jarosite Formation This operation eliminates iron from the solution by forming jarosite which is filtered and taken out of the circuit. During jarosite precipitation, other impurities such as arsenic, antimony, indium, selenium and tellurium are coprecipitated and are also eliminated from the circuit. Jarosite Acid Washing This stage consists of six reactors which help extract the metals in the calcine that is added as a neutralizing agent in the jarosite formation circuit, in order to increase the overall recovery of zinc. Spent electrolyte is added to obtain a free acidity of 60 g/L and steam is used to maintain a temperature of 92°C. Sulphates Purge The purpose of this circuit is to bleed sulphates from the circuit; however, it also bleeds other salts such as manganese and magnesium sulphates, as well as chlorides and fluorides, to acceptable operating levels. The sulphates bleed is important because it allows an increased addition of sulphuric acid to the circuit and this achieves an increase in zinc recovery. Lead – Silver Residue Filtration This stage is used to recover the lead and silver contained in the zinc concentrates and also contributes to the solids balance in the leaching circuit. The filtration of this residue, with adequate washing, also helps to recover the soluble zincin the filter cake. Jarosite Filtration The purpose of this stage is to remove the jarosite to balance the solids and reject impurities from the circuit. It also increases the overall zinc recovery by recovering the soluble zinc as wash liquor from the filter cakes. Jarosite Neutralization The jarosite coming from the filters is neutralized with mixture of Ca(OH)2 and CaCO3 to produce a physically stable material that can be stockpiled and to stabilize any minor metals content that could possibly be leached from the jarosite by rain and subsequently reach the water table. Year after year quality concentrates decrease ,and the Met-Mex Peñoles Zinc Plant treats concentrates with high iron ,cobalt,silica,iron ferrous contents to produce a calcine with 11.5 -12.5 % Fe and 0.018 to 0.030 % Co. Table 1 shows the typical analysis of the calcine treated. Table 1 - Typical analiysis of the Peñoles calcine Element Average Content Range (wt %) (wt %) Zn 57 56 - 58 Fe 12 11.5 - 12.5 Pb 1.0 0.8 - 1.20 Cu 1.20 1.00 - 1.40 Fe ++ 1 0.8 - 1.2 Cd 0.42 0.4 - 0.44 Insol. 2.0 - 4.0 3 Co 0.24 0.018 - 0.30 Ni 0.004 0.003 - 0.007 To get treat high iron calcines , Next Improvements were done in Metalurgica Met-Mex Peñoles Zinc Plant . PROCESS IMPROVEMENTS 1.- Increase Acid free in Weak Acid Leaching from 10 to 20 gr/l . 2.- Increase Acid Free in Hot Acid Leaching from 130 to 160 gr/l . 3.- Increase Retention Time in Jarosite Precipitation from 6 to 7.5 hours . 4.- Flows Balance . 5.- PH Automatic Control in Fourth Purification Reactor . 6.- PH Automatic Control in Jarosite First Reactor . 7.- Increase Temperature in Jarosite Reactors from 88° C to 92° C . 8.- Increase Jarosite Filtration. 9.- Increase Thickening Surface in Acid Wash Jarosite. 10.- Increase Oxidation. 1.- Increase Acid free in Weak Acid Leaching from 10 to 20 gr/l : We Install a Decanter ( D-1) in underflow of Neutral Thickeners 32 and 33 to separate neutral solution/ solids . 2.- Increase Acid free in Hot Acid Leaching from 130 to 160 gr/l : Reduce water in reagents preparation to increase spent flow. 3.- Increase Retention Time in Jarosite Precipitation from 6 to 7.5 hours . We install Reactor # 6 (320 m3) in head-board of jarosite stage. 4.- Flows Balance . Implemented Automatic Control Flows * and get best performance in chemical reactions. 5.- PH Automatic Control in Fourth Purification Reactor . We achieve increase efficiency leaching calcine . 6.- PH Automatic Control in Jarosite First Reactor . We got increase iron precipitation in jarosite stage . 7.- Increase Temperature in Jarosite Reactors from 88° C to 92° C . Install Reactor # 6 in Jarosite stage whit automatic heating system. 8.- Increase Jarosite Filtration. We change one silver-lead press filter to jarosite filtration . 9.- Increase Thickening Surface in Acid Wash Jarosite stage . We enable big one 400 m2 thickener to jarosite acid wash stage . 10.- Increase Oxidation. We install Neutral leaching Reactor # 35 and Jarosite Reactor # 6 both whit Oxidation System ( oxigen) (by Outotec inc.) to oxidize iron ferrous to iron ferric . In the Figure 2 are indicated each one improvements that were done in the diferentes stages of Leaching Process to can treat high iron calcines Figure 2 – Low Silver Leaching circuit Flow Diagram Because of the high impurity contents, several reactors are used for jarosite precipitation. The jarosite precipitation circuit is arranged with nine reactors tanks (see Figure 2). The process is initiated when the solution rich in Fe (15 to 30 g/L) coming from the Weak Acid Leaching each thickener overflow is fed to the first reaction tank # 6. Here calcine is added to neutralize the acid. In reactor # 6, ammonia (4.0 to 5.0 g/L) is added to form the jarosite, and the solution in the reactors is heated with steam to a temperature of 92°C. Additional calcine is added in reactors # 7 and 8 to continue lowering the acid content (see Table 2). Table 2 - Typical process variables values in jarosite precipitation circuit Variable Average Content Range (gr/l) (gr/l) Fe (Reactor 6) inlet 20 15 - 25 Fe (Reactors 28 and 32 ) outlet 6 4-8 Acidity (Reactor 6 ) 22 15 - 30 NH3 (Reactor 6) 4.5 4-5 The reactions for jarosite formation are: 2 NH3+ 2 H2O 2 NH4OH 3 Fe2(SO4)3+ 10 H2O + 2 NH4OH (1) (NH4)2Fe6(SO4)4(OH)12+ 5 H2SO4 (2) The formed jarosite is sent to three thickeners for sedimentation. The overflow is a zinc solution containing 4-8 g/L Fe, and this is sent to the Fourth Purification stage to recover the zinc and to make use of the iron in solution to precipitate any remaining impurities. Jarosite from the underflow of the thickeners is sent to the acid wash stage, to leach the calcine that was added for acid control in the jarosite circuit. After acid washing, the washed jarosite is sent to thickener No. 35. The overflow of thickener No. 35 is returned to the jarosite precipitation circuit because of its high iron content, and the underflow is sent to two washing thickeners (No. 34 and No. 134) to lower the soluble metals content. The overflow of these thickeners is sent to the Jarosite Thickeners to help to clarify them, and the slurry of the underflow is pumped to six press filters . The jarosite is neutralized with mixture of Ca(OH)2 and CaCO3 to stabilize the heavy metals and to give the residue the proper physical characteristics to be transported by truck to the outside plant storage areas. A typical jarosite analysisis shown in Table 3. Table 3 - Typical analisis of the Peñoles jarosite Element Average Content Range (wt %) (wt %) Zn 3.5 03-abr Fe 27 26 - 28 Pb 1.7 1.5 - 2.3 Cu 0.4 0.3 - 0.5 S 13 12 - 14 Cd 0.18 0.16 - 0.20 Insol. 5.5 5.2 - 5.8 Ar 150 140 - 160 Co 0.007 0.004 - 0.01 Ni 0.004 0.002 - 0.006 REMOVAL OF IMPURITIES DURING JAROSITE PRECIPITATION The jarosite precipitation stage is very important in the leaching process because most of the iron from the solution, as well as a considerable amount of the impurity content, is eliminated in this circuit, as shown in Table 4. Table 4 - Impurities Removal in jarosite circuit Element (Reactor 6) inlet (Reactors 28 - 32) outlet (mg/l) (mg/l) Ar 550 150 Sb 41 26 Se 17 12 Te 32 25 Si 879 414 Sn 4.86 3.22 In 48.4 20.36 Cl 355 284 F 46 42 Mn 14530 14180 The iron precipitated as jarosite also serves as a purge and helps reduce the recirculation of impurities that have to be removed in the Fourth Purification circuit and in the hot and cold purification stages in the purification area. Tests have shown that in the Peñoles jarosite circuit, the amount of iron removed in the process correlates with the amountof impurities removed (arsenic, antimony, indium, etc.) There is also a direct relation with the variables that are important for the jarosite formation and the amount of iron precipitation. The correlation between iron removal and impurities elimination is shown in previous studies (see REFERENCES). The most important variables for jarosite precipitation are temperature, acid concentration of the solution andthe ammonia concentration.These variables are plotted against the amount of iron precipitated in Figures 8 to 10, respectively. For an efficient precipitationofjarosite these variables must be closely monitored and controlled within specific ranges. CONCLUSIONS Year after year around of world quality concentrates are decrease ,is dificult to find good concentrates,Iron,Silica,Lead,Cooper ,Iron Ferrous,Germanium are increase,causing problems at normal operationes ,is necesary to continue improvements the process to achieve daily production to meet customer requirements . REFERENCES 1. G. Garcia and C. Valdez, “Jarosite Disposal Practices at the Peñoles Zinc Plant”, Iron Control and Disposal, J.E. Dutrizac and G.B. Harris, Eds., Canadian Institute of Mining, Metallurgy and Petroleum, Montreal, Canada, 1996, 643-650. 2. C. Rocha and J. Ayala , “Impurity Removal in the Jarosite Precipitation Circuit of the Electrolytic Zinc Plant of Met-Mex ,Peñoles S.A de C.V” . Iron Control Technologies, J.E Dutrizac and Patricio Riveros Eds., CANMET Mining and Mineral Sciences Laboratories Ottawa Canada,2006 , 501-512 .