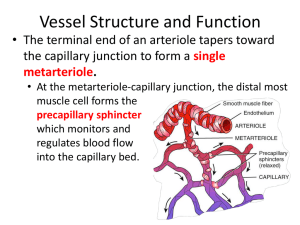
Colloid Wick Lab Write-Up
advertisement

Colloids Laboratory Wicking Flow in Porous Media Performed: 10/27/10 & 11/3/10 Written By: John Willey Group Members: Andrew Adams Ian Gregg Abstract The goals of this experiment were to determine the effect of capillary and gravitational forces on several fluids, to test the Washburn equation with experimental data, to measure the capillary action or the penetration of filter paper and to design a wicking flow assay. The data fits the Washburn equation for capillary flow at different inclines. For wicking flow it was found that the Washburn equation does not fit the data accurately in most cases. When dye penetrated into a packed porous media it was found that the dye moved at a constant velocity. In conclusion it is found that the Washburn equation predicts the capillary action in capillaries better than it does for fluid penetration of a porous media, and when designing a wicking lateral flow assay the movement of an analyte in a packed porous media can be approximated by a constant velocity. Introduction There is a large need for testing assays that are cheap, fast and reliable. Lateral wicking assays are a method of testing that meets all three of these criteria. Assays detect the presence of a particular protein or another particular chemical species that would indicate a specific condition is present. A famous example is the home pregnancy test, but there are others on the market as well that test for different diseases. To design a wicking assay, it is required to know how fast and how far different analytes will wick along a piece of filter paper. This is because many tests require several different chemical reactions to occur at different stages and the designer must know where to put these stages on the wick. This experiment allowed the study of fluid penetration in wicks and also considered the use of the Washburn equation to predict distance covered by a fluid over time in both wicks (porous media) and capillary tubes. Understanding fluid movement in different conditions will be applicable in the design of wicking flow assays. Capillary Figure 1: capillary tube diagram X α Fluid Trough Theory Capillary forces exist because of surface tension forces that act on a fluid. This force can be modeled by the Young-Laplace equation. ∆𝑃 = 2𝜎 cos 𝜃 𝑅 Where ∆𝑃 is the pressure generated by the surface tension, 𝜎 is the surface tension of the fluid, cos 𝜃 is the contact angle of the fluid to the capillary wall, and 𝑅 is the mean radius of the capillary. It can be seen that as the radius of the capillary decreases, the pressure generated by surface tension forces increases. If the fluid is static, then there cannot be a pressure difference exerted on the fluid. The force generated by surface tension must be counteracted by another force, usually gravitational forces. If the capillary tube is at an incline, then as the fluid rises in the tube it increases the weight of the fluid in the capillary. Eventually the weight of the fluid will balance against the interfacial forces and the fluid will become static. At this equilibrium the rise height can be calculated from the condition that the net force on the fluid is equal to zero. 𝑋= 2𝜎 cos 𝜃 𝜌𝑔𝑅 sin 𝛼 Where 𝜌 is the fluid density, 𝑔 is the gravity constant, and sin 𝛼 is from the incline of the capillary tube. If the fluid is not in equilibrium the Washburn equation can be used to model the distance traveled by the fluid over time. If sin 𝛼 = 0 𝜎 cos 𝜃 𝑅 1/2 1/2 𝑘𝑤 = ( ) ∗𝑡 2µ If sin 𝛼 ≠ 0 𝑡= 8𝜇𝑋 𝑥 𝑥 (− ln (1 − ) − ) 2 𝜌𝑔𝑅 𝑋 𝑋 Where 𝜇 is the viscosity of the fluid 𝑘𝑤 is the Washburn slope and 𝑋 is the total distance traveled by the fluid in the capillary tube. This experiment will test the validity of these equations for capillary tubes, and will test if they can be applied to wicking or fluid penetration of a porous media successfully. Experimental The experiment was performed in three separate parts, capillary flow, wicking and designing a lateral flow wick assay. To observe capillary flow there were two capillaries prepared, one had a volume of 10 micro liters and the other had a volume of 5 micro liters. These capillary tubes were set at different incline angles in two different fluids. The fluids were de-ionized water and silicon oil. The distance traveled by the fluid in the capillary was measured over time, along with the equilibrium rise height when α = π/2 radians. Wicking experiments were performed on nitrocellulose and whatman 41 filter paper. The paper was cut into thin strips and allowed to dip into de-ionized water or silicon oil inside of a test tube. The tube containing water was covered by a damp cloth, to prevent evaporation from the wick. The distance traveled by the fluid on the wick was measured over time. To design a lateral flow wick assay we measured the time required for both water and a dye to travel along the wick. While designing the wick assay we used a pattern with varying lengths of wick that met at a common point. The assay had four loading pads for the chemical species and one sink pad, to encourage analyte movement. The assay was treated with a pH sensitive dye, and a dilute acid and base were then loaded onto separate parts of the wick assay. Results A. Capillary Rise Table 1: Theoretical Equilibrium Rise Height 10 micro liter capillary 5 micro liter capillary Ethylene Glycol 3.5 cm 4.9 cm Silicon Oil 1.7 cm 2.5 cm Table 2: Measured Equilibrium Rise Height 10 micro liter capillary 5 micro liter capillary Ethylene Glycol 3.6 cm 5.2 cm Silicon Oil 1.9 cm 2.7 cm Comparison of Measured to Theoretical Rise Height Uncertainty in Height Measurements: 0.25 cm √(0.25𝑐𝑚 ∗ 0.25𝑐𝑚) ≈ |(5.2𝑐𝑚 − 4.9𝑐𝑚)| 0.25 ≈ 0.3 Thus the difference between the measured and experimental data is on the same order of magnitude. The theoretical rise height agrees with measured values. Table 3: Measured Contact angle Cos (θ) 10 micro liter capillary 5 micro liter capillary Ethylene Glycol 1.04 1.05 Silicon Oil 1.09 1.09 Another demonstration that the equilibrium rise height agrees with the experimentally observed rise height is that the experimental value of the cosine of the contact angle (cos 𝜃) is approximately one, which is expected when calculating the theoretical value. Figure 2: plot of distance traveled by ethylene glycol over time1/2 in a 10 µL tube Distance Travelled (m) Ethylene Glycol in a 10 microLiter capillary 0.12 0.1 0.08 0.06 0.04 Experimental (alpha = 0) 0.02 Washburn (alpha = 0) 0 0 2 4 6 8 SQRT[ Time Elapsed (s) ] The Washburn slope agrees with the experimental slope when Ethylene Glycol was tested in a 10 micro liter capillary tube. The experimental values are shifted downward; this is thought to have occurred due to an initial timing error at the start of the experiment. Figure 3: plot of distance traveled by ethylene glycol over time1/2 in a 5 µL tube Ethylene Glycol in a 5 microLiter capillary 0.14 Distance Traveled (m) 0.12 0.1 Experimental (alpha = pi/2) 0.08 Experimental (alpha = pi/4) 0.06 Experimental (alpha = 0) Washburn (alpha = 0) 0.04 Washburn (alpha = pi/2) 0.02 Washburn (alpha = pi/4) 0 0 2 4 6 8 10 SQRT[ Time Elapsed (s) ] Figure 4: plot of distance traveled by silicon oil over time1/2 in a 10 µL tube Silicon Oil in a 10 microLiter capillary 0.14 Distance Traveled (m) 0.12 0.1 0.08 Experimental (alpha = 0) 0.06 Washburn (alpha = 0) 0.04 0.02 0 0 5 10 SQRT[ Time Elapsed (s) ] 15 20 Figure 5: plot of distance traveled by silicon oil over time1/2 in a 5 µL tube Silicon Oil in a 5 microLiter capillary Distance Traveled (m) 0.12 0.1 0.08 Experimental (alpha = 0) 0.06 Experimental (alpha = pi/4) 0.04 Washburn (alpha = 0) 0.02 Washburn (alpha = pi/4) 0 0 5 10 15 20 SQRT[ Time Elapsed (s) ] The experimental values of silicon oil are compared to the Washburn predicted values in Figures 4 & 5. Silicon oil does not have a pronounced meniscus, causing difficulty when taking the measurement at α = 0. This can be seen from the deviation from predicted values and the experimental values that exist only when α = 0. B. Wicking into Filter Paper Microscopic Observations: It appears from Figure 6 that the diameter of the average pore in nitrocellulose is of the order of 5 microns. It also appears that the pores are finer and more regularly dispersed then the pores found in whatman 41 paper. From Figure 7 the average pore diameter of whatman 41 appears to be on the order of 10 microns, and the pores appear jagged and less uniform then those of Figure 6. The image depicted in Figure 7 appears to be striated and fibrous, while Figure 6 appears soft and clumped. Figure 6: Microscopic view of nitrocellulose paper Figure 7: Microscopic view of whatman 41 paper Figure 8: plot of distance traveled by de-ionized water over time1/2 in nitrocellulose Distance Traveled (m) D.I. Water penetrating Nitrocellulose 0.08 0.07 0.06 0.05 0.04 0.03 0.02 0.01 0 Experimental Washburn 0 5 10 15 20 SQRT[ Time Elapsed (s) ] The experimental values of de-ionized water appear to penetrate at the rate predicted by the Washburn equation. The initial kink in the experimental values at start up might be attributed to initial wetting of the filter paper or experimental error due to timing. Figure 9: plot of distance traveled by de-ionized water over time1/2 in Whatman 41 D.I. Water penetrating Whatman 41 Paper Distance Traveled (m) 0.12 0.1 0.08 Experimental 0.06 Washburn x = 0.003 t1/2 + 0.011 0.04 Linear (Experimental) 0.02 Linear (Experimental) 0 0 5 10 15 SQRT[ Time Elapsed (s) ] 20 25 The experimental data for de-ionized water penetrating whatman 41 demonstrates a dependence on the square root of time. This is similar to the Washburn equation, except the data is fit best by a line with a non zero intercept. This must be due to experimental error because the fluid cannot penetrate the paper until after the experiment has started. Figure 10: plot of distance traveled by silicon oil over time1/2 in Nitrocellulose Distance Traveled (m) Silicon Oil penetrating Nitrocellulose 0.16 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 Experimental Washburn x = 0.004t + 0.018 R² = 0.994 0 5 10 15 Linear (Experimental) 20 SQRT[ Time Elapsed (s) ] Figure 11: plot of distance traveled by silicon oil over time1/2 in Whatman 41 Distance Traveled (m) Silicon Oil penetrating Whatman 41 Paper 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 Experimental x = 0.003t + 0.010 R² = 0.994 0 5 10 15 SQRT[ Time Elapsed (s) ] 20 Washburn Linear (Experimental) 25 Silicon oil penetrating nitrocellulose (Figure 10) appears to follow a linear line on a plot relating the square root of time to the distance traveled. The Washburn equation also shares the same dependence on time, although the slopes and intercepts are different. Silicon oil penetrating whatman 41 paper (Figure 11) appears to have a linear dependence on time. Table 4: measured wicking equivalent pore radius Nitrocellulose Whatman 41 D.I. Water 0.53 µm 2.4 µm Silicon Oil 0.44 mm 0.22 mm The equivalent pore radius is highly dependent on what fluid is using, as the values found for de-ionized water are three orders of magnitude different from those found for Silicon Oil. Table 5: estimated wicking equilibrium rise heights Nitrocellulose Whatman 41 D.I. Water 28 m 6.3 m Silicon Oil 9.6 mm 19 mm It appears that the equivalent radius is smaller for water in the filter paper, which causes the rise height to be higher. This is of course the case, since rise height is inversely proportional to the radius of a capillary in the capillary model is being utilized to describe the wicking paper. C. Lateral Flow Assay Figure 12: moving front of de-ionized water on the lateral assay over time1/2 Distance Traveled (cm) D.I. Water penetrating Wick Assay 6 5 4 x = 0.360 t1/2 R² = 0.989 3 2 1 0 0 2 4 6 8 10 12 14 SQRT[ Time Elapsed (s) ] Flow through the packed membrane appears to follow the Washburn format, having the same shape as the function x = kW t1/2. The constant kW is the Washburn slope, and depends on the fluid and membrane properties. From Figure 12 the data has been fit to the Washburn format which appears as a linear curve. The Washburn slope in this case would be 0.36 (cm/s) Figure 13: moving front of dye on the lateral assay over time1/2 Dye penetrating packed Wick Assay, t1/2 Distance Traveled (cm) 5 4 3 x = 0.174 t1/2 R² = 0.953 2 1 0 0 5 10 SQRT[ Time Elapsed (s) ] 15 20 25 Figure 14: moving front of dye on the lateral assay over time Distance Traveled (cm) Dye penetrating packed Wick Assay, t 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 x = 0.007t + 0.730 R² = 0.992 0 50 100 150 200 250 300 350 400 450 500 Time Elapsed (s) The dye does not appear to follow the Washburn format, as can be seen by the attempted fit on Figure 13. The distance traveled by the dye appears to be proportional to time, not time 1/2. Since the Washburn format is x = kW t1/2, it cannot be followed by the wicking dye as demonstrated by the data collected. Apparently the dye travels in a linear fashion, as shown by the fit line in Figure 14. Figure 15: diagram of designed lateral flow assay, made of nitrocellulose Loading Pad Sink Pad Figure 16: wicking assay created, snapshots taken over time Time The diagram of the lateral flow wicking assay in Figure 15 was generated and is shown in Figure 16. A pH sensitive dye was placed on the test, which initially is yellow. After that an acidic solution was applied to two loading pads, along with a basic solution (on separate pads). The purple pads are the recipients of the acidic solution, while the light yellow pads are the recipients of the basic solution. The larger pad that is only partially yellow is the sink for the experiment. As can be seen, the acidic solution wicked towards the center of the assay and was unable to continue. This is due to the basic solution counteracting the acid, preventing the acid from activating the dye past the center of the wicking assay. Sample Calculations Radius of 5 µL capillary 𝑟 = √𝑉𝑜𝑙𝑢𝑚𝑒/𝑙𝜋 1,000𝑐𝑚3 √ 𝑟= = 0.0177𝑐𝑚 5.1𝑐𝑚 ∗ 𝜋 Theoretical equilibrium rise height ℎ= 2𝜎𝑐𝑜𝑠𝜃 𝜌𝑔𝑟 𝑓𝑜𝑟 𝑠𝑖𝑙𝑖𝑐𝑜𝑛 𝑜𝑖𝑙 𝑖𝑛 𝑡ℎ𝑒 5 𝜇𝐿 𝑐𝑎𝑝𝑖𝑙𝑙𝑎𝑟𝑦 𝑁 ℎ= 2 ∗ .0208 𝑚 ∗ 1 𝑘𝑔 𝑚 965 𝑚3 ∗ 9.81 𝑠2 ∗ 0.000177𝑚 = 2.5 𝑐𝑚 Contact Angle cos 𝜃 = ℎ 𝜌𝑔𝑟/2𝜎 cos 𝜃 = 0.027𝑚 ∗ 965 𝑘𝑔 𝑚 0.000177𝑚 𝑘𝑔 ∗ 9.81 2 ∗ (0.0483 )−1 = 1.09 3 𝑚 𝑠 2 𝑚𝑠 Washburn slope for α = 0 𝑘𝑤 = √𝑟 ∗ 𝜎 ∗ 𝑐𝑜𝑠𝜃/2𝜇 𝑁 𝑘𝑤 = √0.000177𝑚 ∗ 0.0208 ∗ 𝑚 1.09 𝑘𝑔 2 ∗ 0.0483 𝑚 𝑠 = 0.0077 𝑚 𝑠 .5 Washburn Equation when capillary tube is horizontal 𝑥 = 𝑘𝑤 ∗ 𝑡 .5 𝑥 = 0.0077 𝑚 ∗ 1.45.5 𝑠 .5 = 0.0093 𝑚 𝑠 .5 Washburn Equation when capillary tube has rise 𝑡= 8𝜇𝑋 𝑥 𝑥 (− ln (1 − ) − ) 𝜌𝑔𝑟 𝑋 𝑋 .5 𝑘𝑔 𝑡 .5 = [ 8 ∗ 0.048 𝑚 𝑠 0.032 𝑚 𝑘𝑔 𝑚 965 𝑚3 9.81 𝑠2 0.000177 𝑚 (− ln (1 − 0.01𝑚 0.01𝑚 )− )] = 1.61 𝑠 .5 0.032𝑚 0.032𝑚 Equivalent wicking radii 𝑟= 8𝜇𝑋 𝑥 𝑥 (− ln (1 − ) − ) 𝜌𝑔𝑡 𝑋 𝑋 𝑘𝑔 𝑟=[ 8 ∗ 0.048 𝑚 𝑠 0.032 𝑚 𝑘𝑔 𝑚 965 𝑚3 9.81 𝑠2 0.6 𝑠 (− ln (1 − 0.01𝑚 0.01𝑚 )− )] = 0.1 𝑐𝑚 0.032𝑚 0.032𝑚 This was done for all times and distances recorded, the final radii found are averages. Discussion Questions: 1) The deviations that occurred from observed capillary flow and theory are likely to have resulted from experimental error in timing the fluid movement. The largest deviations occurred when the capillary tubes are parallel to the lab table. This is likely due to the fact that it is harder to time the fluid in this configuration, especially when considering the first several points. Secondly the contact of the capillary tube with the fluid meniscus may have been partial, which would cause dampened wicking at least initially. 2) In order to find the tortuosity of the different wicking papers, it is important to keep the different fluid types in mind, as the equivalent radii depend greatly on the fluid properties. Table 4 is expanded below to evaluate the tortuosity of the pore channels. In order to do so, the actual pore radius must be acquired, and this is roughly discernable in Figures 6 and 7 as shown in the results. The pore radius of nitrocellulose is approximately 2.5 micrometers, and that for whatman 41 is found to be approximately 5 micrometers. 𝜏 = 𝑡𝑜𝑟𝑡𝑢𝑜𝑠𝑖𝑡𝑦 = 𝑝𝑜𝑟𝑒 𝑟𝑎𝑑𝑖𝑢𝑠 𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 𝑟𝑎𝑑𝑖𝑢𝑠 [Berg] As is visible from Table 6, the tortuosity of silicon oil is less than that of de-ionized water by two or three orders of magnitude, depending on the paper used. This implies that while the pore sizing and regularity affect how well a fluid wicks into a material, the fluid properties actually have a greater effect on how well that fluid wets or wicks a given material. Table 6: Evaluation of tortuosity of nitrocellulose and whatman 41 Nitrocellulose paper Whatman 41 paper Equivalent radii Tortuosity Equivalent radii tortuosity D.I. Water 0.53 µm 4.72 2.4 µm 2.08 Silicon Oil 0.44 mm 0.00568 0.22 mm 0.0227 3) The differences of the equivalent radii for silicon oil and de-ionized water vary greatly for both nitrocellulose and whatman 41 papers. There are several possible reasons that account for this occurrence in the data. Silicon oil and water have a similar volatility [Berg], which means that the oil wicking experiment should have been covered with a damp Chem-Wipe as the water wicking experiment was. It is likely that the error generated by this mistake is not large enough to account for the deviation seen in Table 6 for the effective radii calculated when either de-ionized water or silicon oil was used. Since the actual pore radius of the filter paper is constant it is the fluid that changes in how it interacts with the paper. Since the Washburn equation is calculating different effective radii for each fluid there must be a fluid property that is not accounted for by the Washburn equation. There may be an interaction that occurs between one of the fluids and the filter paper that does not occur for the other fluid. This would potentially explain the difference observed in the data. 4) Heat pipes utilize wicking to carry a fluid from a condenser to a source of heat. The heat causes evaporation of the fluid, which is carried by either diffusion or pumped towards the condenser. The heat pipe makes use of the latent change of heat between the liquid and gas phase when removing heat from the heat source. The advantages to the heat pipe are that they are cheap and effective. The disadvantages to using a heat pipe are the size limitations and that the operating temperature greatly affects the efficiency of the wicking material and fluid. This second reason is a disadvantage if the operating temperature were to change, because the fluid or wicking material may have to change as well. Sources of Error The largest sources of error in this experiment were due to human reflex and coordination. The experiments occurred in a rapid fashion, and our group experienced difficulty starting the time at the exact moment that the wicking paper touched the fluid. This was also a difficulty with the capillary tubing. When the capillary tube was parallel to the meniscus, it was difficult to identify the meniscus within the capillary tube until the fluid had traveled 1-3 cm. Error appears to be the most significant in the experiment with Ethylene Glycol in the 10 µL capillary tube (Figure 2). This is also visible when de-ionized water is used to wick a flow assay, (Figure 13). The initial timing is off by several seconds in most trials as demonstrated by the fact that the experimental data does not show wicking to be zero before the experiment occurs. In another words, on all of the plots that use wicking or capillary flow, the data should pass through the origin. An error in experimental setup arose in the wicking portion of the experiment. When deionized water was used, the top of the test tube containing the fluid and the wick was covered with damp paper due to the volatility of the water. Accordingly, this should have been performed for the silicon oil as well, as the literature sates that water and silicon oil both have similar volatilities [Berg]. Analysis of Results Capillary action in capillary tubes fit the Washburn equation as demonstrated in Figures 2, 3, 4 & 5. In Figure 2 the experimental data parallels the Washburn equation, but it is also shifted downward from what is expected. This is believed to occur due to experimental error in timing the motion of the fluid. In Figure 3 there is a lot of overlap between the values predicted from theory and from the experimental values. One issue encountered in Figure 3 & 5 is that the value of X was unknown. The equilibrium rise height is known for when the capillary is perpendicular to the ground, but not when it is at another incline angle. To resolve this issue, a value of X was chosen that was less than 2 cm greater than the largest distance traveled by the fluid in a particular capillary at the specified incline. From figure 2, 4 & 5 the experimental values for a capillary parallel to the ground are seen to deviate from the Washburn predicted values. This is likely due to experimental error caused by difficulty in timing the initial distance traveled by the fluid, or by the fact that the capillary might have been at a slight and unrecorded incline. The literature states that fluid movement in filter paper is well described by the Washburn Equation [Berg], but the results from this experiment disagree. Wicking flow experimental values are compared to the theoretical values predicted by the Washburn equation in Figures 8, 9, 10 & 11. The data only fits the predicted values in one case, and that is when de-ionized water wicks into nitrocellulose as is visible in Figure 8. Examination of Table 6 shows that the tortuosity of whatman 41 and nitrocellulose are of the same order of magnitude for de-ionized water. It might be expected then that de-ionized water would wick both papers as predicted by the Washburn equation. From Figure 9 it can be seen that this is not the case, either due to experimental error or an effect of the pore size and shape of the whatman 41 paper upon deionized water. When silicon oil is used instead of water, wicking is not described by the Washburn equation regardless of the paper used as is demonstrated in Figures 10 & 11. In fact, the wicking of either paper with silicon oil is better described by a first order dependence on time then by the Washburn format. It shall be noted here that the experiment performed for silicon oil and nitrocellulose and the whatman 41 filter paper may end with different results if the test tube were instead covered by a damp cloth, to prevent evaporative losses from the wick. It is likely that this error is to blame for the disagreement for the discrepancy between the Washburn format and the experimental data. This is because of the fact that the wicking values are consistently less than those predicted by the Washburn equation. If evaporative losses were prevented, then the rate of wicking would increase. The Washburn equation cannot take into account the evaporation that occurs during the exercise, and would therefore not be able to predict the wicking rate of silicon oil observed. The best way to test this would be to perform the same experiment with wicking paper and silicon oil as performed before, but with the exception of covering the test tube with a cloth dampened with silicon oil. The Washburn equation takes surface tension and viscosity into account, which implies that a different fluid property is responsible for the differences between water and silicon oil when wetting nitrocellulose. I hypothesize that this difference is due to an interaction that occurs between the de-ionized water and the nitrocellulose, and that this interaction does not occur between silicon oil and the nitrocellulose. In all but one case the actual wicking values are lower than those predicted by the Washburn equation, which means that wicking paper is not as efficient as a single small capillary, perhaps due to the papers tortuosity. A single capillary is a straight shot, but a piece of filter paper is a maze of broken capillaries. It therefore may be expected that filter paper will not wick as well as a small capillary, and this is demonstrated by the disagreement between Washburn equation and the wicking data in most cases. The movement of dye across a lateral piece of packed wicking paper is not described by the Washburn equation, but rather a linear fit. The velocity appears constant and depends only on a first order relationship to time. This is not the case for the movement of water across the same wick when the wick is dry. Figure 12 demonstrates the fact that de-ionized water wicks nitrocellulose paper with a dependence on the square root of time, or in other words in the Washburn format. This is in agreement with the results in Figure 8. This means that the dye does not move at a constant velocity because of the wicking paper itself, otherwise the water would also demonstrate a constant velocity. I hypothesize that the dye’s movement in the wicking paper would be better described by a counter-diffusion model then the Washburn equation. This is supported by the fact that the water wicks the paper at different time dependence then the dye does. If the dye displacement is described by an equal molar counterdiffusion model, then the geometry of the wick will affect the rate of diffusion. This means that an additional experiment could be performed with a disk cut out of nitrocellulose. It could be wetted, and then the dye placed into the center of the disk. If the displacement of the dye is a factor of 2πr (the perimeter of the circle covered by the dye) less than when the experiment is performed with a straight wick, then it is likely that the diffusion model will describe the displacement of the dye over time. Conclusion From the results of this experiment two things can be concluded. The first is that capillary action in capillary tubes is generally described by the Washburn equation. The second is that wicking flow is not generally described by the Washburn equation, but instead depends on the wicking material structure, and on any interactions that may occur between the fluid and the wicking material. References Berg, J. C. (2010). An Introduction to Interfaces & Colloids: The Bridge to Nanoscience. New Jersey: World Scientific (pp. 55, 284-289)