Clinical effects of technOlogical iMProvements with new
advertisement
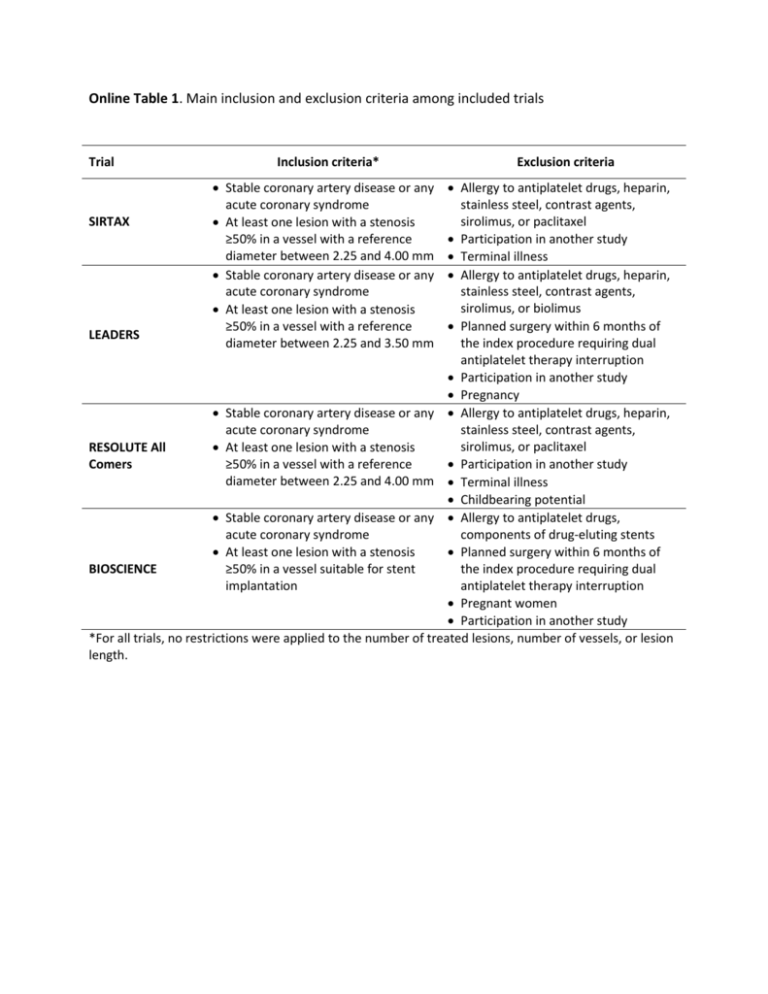
Online Table 1. Main inclusion and exclusion criteria among included trials Trial Inclusion criteria* Stable coronary artery disease or any acute coronary syndrome At least one lesion with a stenosis ≥50% in a vessel with a reference diameter between 2.25 and 4.00 mm Stable coronary artery disease or any acute coronary syndrome At least one lesion with a stenosis ≥50% in a vessel with a reference diameter between 2.25 and 3.50 mm Exclusion criteria Allergy to antiplatelet drugs, heparin, stainless steel, contrast agents, sirolimus, or paclitaxel SIRTAX Participation in another study Terminal illness Allergy to antiplatelet drugs, heparin, stainless steel, contrast agents, sirolimus, or biolimus Planned surgery within 6 months of LEADERS the index procedure requiring dual antiplatelet therapy interruption Participation in another study Pregnancy Stable coronary artery disease or any Allergy to antiplatelet drugs, heparin, acute coronary syndrome stainless steel, contrast agents, sirolimus, or paclitaxel RESOLUTE All At least one lesion with a stenosis Comers ≥50% in a vessel with a reference Participation in another study diameter between 2.25 and 4.00 mm Terminal illness Childbearing potential Stable coronary artery disease or any Allergy to antiplatelet drugs, acute coronary syndrome components of drug-eluting stents At least one lesion with a stenosis Planned surgery within 6 months of BIOSCIENCE ≥50% in a vessel suitable for stent the index procedure requiring dual implantation antiplatelet therapy interruption Pregnant women Participation in another study *For all trials, no restrictions were applied to the number of treated lesions, number of vessels, or lesion length. Online Table 2. SYNTAX score in patients with diabetes, single-, and multi-vessel percutaneous coronary intervention Diabetes (n =1,310) SYNTAX score ≤22 23-32 >32 13.9±8.8 1090 (83.2%) 178 (13.6%) 42 (3.2%) PCI: Percutaneous coronary intervention. Single-vessel PCI Multi-vessel PCI (n =4,725) (n =1,353) 11.8±8.1 4224 (89.4%) 404 (8.6%) 97 (2.1%) 17.7±9.2 991 (73.2%) 272 (20.1%) 90 (6.7%) Online Table 3. Cox-regression analysis for the interaction between the type of drug-eluting stent and the SYNTAX score HR (95%CI) p-value 0.76 (0.64-0.89) <0.001 1.72 (1.46-1.95) <0.001 New-generation vs. early-generation DES 0.54 (0.44-0.68) <0.001 SYNTAX score* 1.73 (1.39-2.06) <0.001 New-generation vs. early-generation DES 0.36 (0.23-0.56) <0.001 SYNTAX score* 2.08 (1.34-3.02) <0.001 Cardiac death, myocardial infarction, or ischemia-driven TLR New-generation vs. early-generation DES SYNTAX score* Ischemia-driven TLR Definite stent thrombosis Hazard ratios (HR) with 95% confidence intervals (CI) are derived from Cox-regressions in which the SYNTAX score was used as covariate and the type of drug-eluting stent (DES) as a factor. *The SYNTAX score was log transformed before the analyses (ln[SYNTAX score+1]). The interaction between the type of DES (new- vs. early-generation DES) and the SYNTAX score was not significant for the composite of cardiac death, myocardial infarction, or ischemia-driven target-lesion revascularization (TLR) (p =0.16), ischemiadriven TLR (p =0.25), and definite stent thrombosis (p =0.11). Online Table 4. Cox-regression analysis for the interaction between the type of new-generation drug-eluting stent and the SYNTAX score Crude Analysis Multivariable HR (95%CI) p-value Cardiac death, MI or clinically-indicated TLR Everolimus-eluting stent Adjusted Analysis interaction p-value** Multivariable HR (95%CI) p-value 0.961 1.00 (Ref) 0.853 1.00 (Ref) Resolute zotarolimus-eluting stent 0.95 (0.75-1.20) 0.680 0.96 (0.75-1.22) 0.726 Biolimus-eluting stent 0.95 (0.72-1.24) 0.702 0.92 (0.70-1.21) 0.546 Orsiro Sirolimus-eluting stent 0.79 (0.60-1.02) 0.074 0.80 (0.61-1.05) 0.113 SYNTAX score* 1.61 (1.40-1.87) 0.000 1.52 (1.31-1.77) 0.000 Clinically-indicated TLR Everolimus-eluting stent 0.143 1.00 (Ref) 0.167 1.00 (Ref) Resolute zotarolimus-eluting stent 1.05 (0.75-1.49) 0.770 1.02 (0.72-1.44) 0.929 Biolimus-eluting stent 1.20 (0.83-1.75) 0.332 1.13 (0.77-1.66) 0.534 Orsiro Sirolimus-eluting stent 0.93 (0.63-1.36) 0.706 0.94 (0.63-1.39) 0.755 SYNTAX score* 1.60 (1.29-1.98) 0.000 1.53 (1.23-1.91) 0.000 Definite stent thrombosis Everolimus-eluting stent interaction p-value** 0.579 1.00 (Ref) 0.623 1.00 (Ref) Resolute zotarolimus-eluting stent 2.41 (1.06-5.51) 0.037 2.62 (1.11-6.14) 0.027 Biolimus-eluting stent 3.98 (1.77-8.97) 0.001 3.95 (1.68-9.27) 0.002 Orsiro Sirolimus-eluting stent 1.13 (0.39-3.31) 0.825 1.03 (0.32-3.35) 0.960 SYNTAX score* 1.51 (0.93-2.45) 0.097 1.40 (0.85-2.32) 0.188 HR: hazard ratio. CI: confidence intervals. MI: myocardial infarction. TLR: target-lesion revascularization. *SYNTAX score: ln-transformed[SYNTAX score+1] before analyses. ** p-value of the interaction between the type of new-generation drugeluting stent and the SYNTAX score if added to each of the three models separately. Crude Hazard Ratios HR (95% CI) and p-values are from Cox Regressions with SYNTAX score (ln-transformed+1) as a covariate and type of new-generation drug-eluting stent as a factor. Adjusted Hazard Ratios HR (95% CI) and p-values are from Multiple Imputation estimated Cox Regressions (20 data-sets using Rubin's rule to combine estimates) with SYNTAX score (ln-transformed+1) as a covariate and type of new-generation drug-eluting stent as a factor, adjusting for baseline variables associated with the primary outcome: age, diabetes, renal failure, previous myocardial infarction. Online Figure 1. Study Overview SIRTAX (n=1,012) LEADERS (n=1,707) RESOLUTE All Comers (n=2,292) BIOSCIENCE (n=2,119) Patients assessed for the initial screening (n=7,130) Exclusion criteria that will be applied: Previous coronary artery bypass graft surgery (n =724) SYNTAX Score not available (n =325) Patients included in the analysis (n=6,081) NEW-GENERATION DRUG-ELUTING STENTS (n=4,554) EARLY-GENERATION DRUG-ELUTING STENTS (n=1,527)