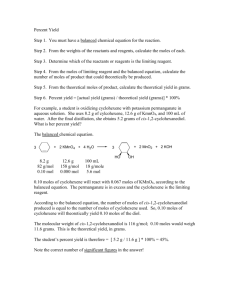
In Class Practice Packet: Answer each of the following problems on
advertisement

In Class Practice Packet: Answer each of the following problems on a separate sheet of paper. You will turn in ALL of your work from this packet at the end of the unit: 1. What is meant by the term “mole ratio”? Give an example of a mole ratio. How is the mole ratio used in solving problems? 2. Consider the reaction represented by the unbalanced equation NH3 + O2 NO + H2O For every 1.00 mol of NH3 that reacts, ____mol of O2 is required. 3. Which would produce a greater number of moles of product: a given amount of hydrogen gas reacting with oxygen gas to make water, or the same amount of hydrogen gas reacting with nitrogen gas to make ammonia (NH3)? Show your work. 4. In the following reaction, what is meant by ‘an excess.’ Oxygen gas is reacted in an excess of hydrogen gas to produce water. 5. The mass of oxygen needed to completely react with 10.0 g of hydrogen to form water is reacted completely with methane (CH4) to form carbon dioxide and water. Determine the mass of carbon dioxide produced. 6. Consider the following unbalanced equation: HCl + MnO2 H2O + MnCl2 + Cl2 You have 5.00 grams of manganese (IV) oxide: a. How many moles of HCl do you need for a complete reaction? b. How many grams of HCl do you need for a complete reaction? c. How many grams of each product (H2O, MnCl2, and Cl2) would be formed? 7. Consider the unbalanced equation: C6H14 + O2 CO2 + H2O. What mass of O2 is required to react with 11.5 g of C6H14? 8. Consider the unbalanced equation: Na(s) + Cl2(g) NaCl(s). a. What mass of NaCl can be produced from 25.0 g of Cl2 and excess Na? b. If there was a 87.6% yield, how much Na was used? 9. How many moles of NaNO3 will be produced from 2.3 moles of Na2CO3 in the following reaction? Na2CO3(aq) + Ca(NO3)2(aq) CaCO3(s) + 2NaNO3(aq) 10. Balance the following reaction. How many moles of PbI2 will be produced from 3.2 moles of KI? Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) 11. Balance the following reaction. If 3 moles of Ca(OH)2 are produced, how many moles of H2O reacted? Ca(s) + H2O(l) Ca(OH)2(s) + H2(g) 12. How many moles of O2 are needed to produce .85 moles of P2O5? P(s) + O2(g) P2O5(s) 13. When .38 moles of aluminum oxide decompose, how many moles of aluminum and how many moles of oxygen will be formed? 14. If .25 moles of aluminum reacts with a copper (II) chloride solution, how many grams of copper will be produced? 15. If 10.0g of zinc reacts with an HCl solution, how many moles of hydrogen gas will be released? 16. How many moles of hydrogen gas are needed to react completely with 15.1g of chlorine gas to produce hydrogen chloride? 17. How many grams of oxygen gas are needed to react completely with 16.2g of hydrogen gas to produce water? How many grams of water will be produced? 18. Gasoline (C8H18) burns in your car engine to form the normal combustion reaction. If 9.0 x 103g of gasoline are burned, how many grams of CO2 are produced? 19. Nitrogen monoxide gas goes through synthesis in your car engine to produce nitrogen dioxide. If 48g of NO reacts completely with oxygen gas, what mass of oxygen gas was needed? 20. How many grams of aluminum are required to produce 410.g of aluminum oxide through a reaction with oxygen gas? 21. Hydrochloric acid (HCl) reacts with calcium carbonate to produce H2CO3 and calcium chloride. If the 16.00 moles of hydrochloric acid and 15.00 moles calcium carbonate are reacted, how much calcium chloride will be produced? How much of your excess reactant will remain? 22. Water is formed through the synthesis of oxygen and hydrogen gas. If 6.7 moles of oxygen gas are combined with 29.5 moles of hydrogen, which is the limiting reactant? 23. Water is formed through the synthesis of oxygen and hydrogen gas. If 35.9 moles of oxygen gas are combined with 36.8 moles of hydrogen, which is the limiting reactant? 24. How many moles of CO2 will be created if 37 g of C3H8OH is combusted? How many grams of oxygen gas are needed for a 100 % yield? 25. If 68.3 grams of hydrogen gas reacts with an excess of oxygen gas, how many grams of water could be produced? How much oxygen is needed? 26. Hydrochloric acid (HCl) reacts with calcium carbonate to produce H2CO3 and calcium chloride. If the 16.00 moles of hydrochloric acid and 15.00 moles calcium carbonate are reacted, how much calcium chloride will be produced? 27. Using the reaction, 4Al + 3O2 2Al2O3, identify the limiting reactant in each of the following a. 0.25mol Al and0 .40mol O2 b. 58.5g Al and 98.0g O2 c. 78.2g Al and 113.1g O2 d. 78.2g O2 and 113.1g Al 28. In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47.2 g Fe. When a careless chemistry student carries out the experiment, the actual yield is 42.9 g Fe. Calculate the percentage yield. Your other product is carbon dioxide. 29. Hexane (C6H14) burns in oxygen to produce carbon dioxide and water. How many moles of oxygen are needed for the complete combustion of 9.88 x 1021 molecules of hexane? 30. Copper metal reacts with a solution of silver nitrate, AgNO3, to produce copper (II) nitrate and silver metal. In carrying out this reaction, a piece of copper wire was immersed in a solution of silver nitrate until the reaction stopped. The original mass of the copper wire was 2.36 grams. After the reaction stopped, the mass of the wire was 1.03 grams. What mass of silver was produced? 31. If 68.3 g of zinc metal are reacted with 92.6 g of lead (II) carbonate, zinc carbonate and lead metal are formed, which reactant will be in excess? Calculate the mass of the excess reactant that will remain. 32. In the reaction between Na and Fe(NO3)3, the theoretical yield of an experiment is calculated to be 47.2 g Fe. When a careless chemistry student carries out the experiment, the actual yield is 42.9 g Fe. Calculate the percentage yield. How many grams of each reactant must be added to create a 100.0% yield? 33. A reaction between hydrazine, N2H4, and dinitrogen tetoxide, has been used to launch rockets into space. The reaction produces nitrogen gas and water vapor shown in the unbalanced equation below. N2H4 (l) + N2O4 (l) N2 (g) + H2O (g) a. What is the mole ration of N2H4 to N2? b. What amount of water will be produced from 14,000 moles of hydrazine used by the rocket? 34. Oxygen gas and solid potassium chloride can be produced by decomposing potassium chlorate. a. Write a balanced equation for the reaction. b. If 125 g of KClO3 is heated and decomposes completely, What amount of oxygen gas is produced? 35. Identify the limiting reactant and the excess reactant in the following situations: a. firewood burning in a campfire b. sulfur compounds from the air tarnishing silver c. NO2 gas reacting with oxygen and water vapor in air to produce acid rain. 36. Hydrochloric acid (HCl) secreted in your stomach can be neutralized in a double replacement reaction by taking an antacid such as aluminum hydroxide. a. Write a balanced equation for the reaction. b. If 34.0g HCl are secreted and 12.0g Al(OH)3 are taken, is there enough antacid to react with all of the acid? 37. Ammonia, NH3, is used throughout the world as a fertilizer. To manufacture ammonia, nitrogen gas is combined with hydrogen gas in a synthesis reaction. If 92.7Kg N 2 and 265.8kg H2 are used, which is the limiting reactant? 38. Coal gasification is a process that converts coal into methane gas. If this reaction has a percentage yield of 85%, how much methane can be obtained from 1.26 g of coal? C(s) + H2O (l) CH4 (g) + CO2 (g) 39. When phosphorous burns in the presence of oxygen, P4O10 is produced. In turn, P4O10 reacts with water to produce phosphoric acid. P4O10 (g) + H2O (l) H3PO4 (aq) a. When 1.00x102 g of P4O10 reacts with 2.00x102g of H2O, what is the theoretical yield of phosphoric acid? b. If the actual yield is 126.2 g of H3PO4, what is the percentage yield for this reaction? 40. What mass of barium nitride (Ba3N2) is produced from the combination reaction between 22.6g solid barium and 4.2g nitrogen gas? a. How much Barium Nitride will be produced? b. What is the limiting reactant? c. What is the excess reactant? d. How much excess reactant will be left?